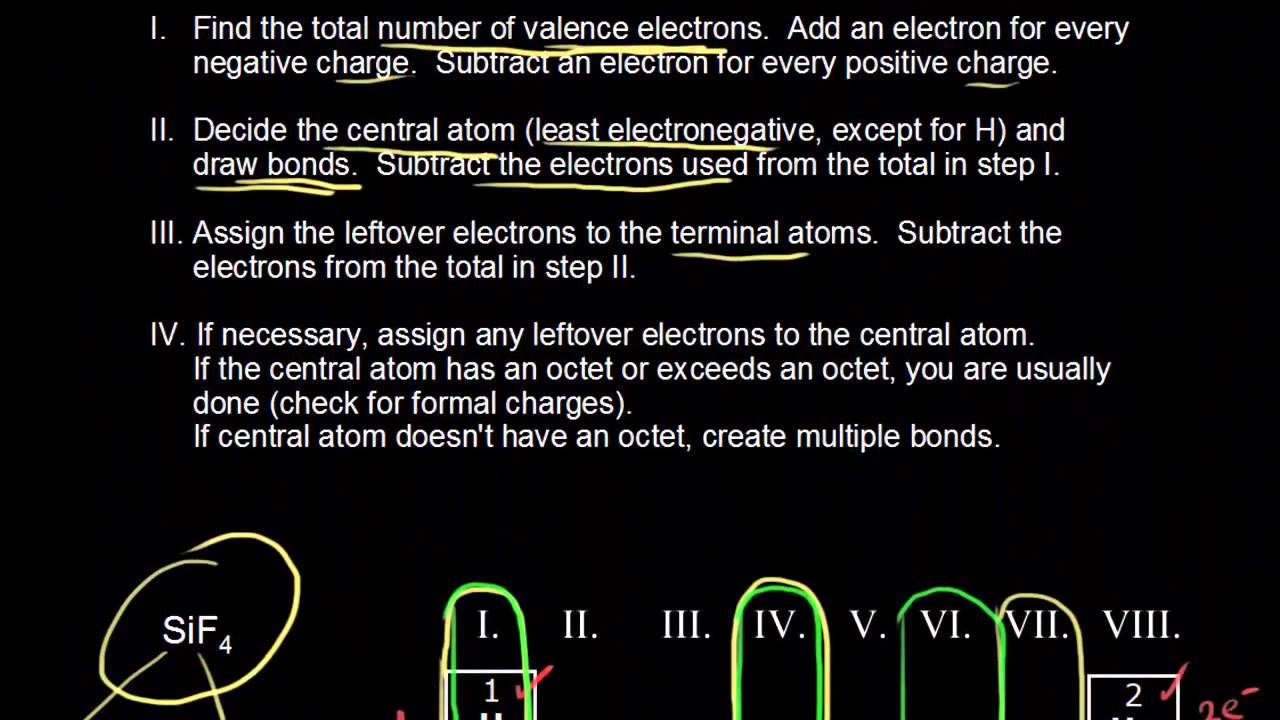
37 draw an electron dot diagram for ammonia
Which of the following is true regarding the Lewis diagram for ammoni a? (2 points) a. Ammonia contains three covalent bonds, one to each hydrogen atom, and the nitrogen atom has no lone pairs of electrons. b. Question: Draw the Lewis electron dot diagram for ammonia (NH3) on a sheet of scrap paper. You may consult the periodic table on Zoom to ... image file c6cc b f2 tif. electron dot diagrams for four simple molecules ammonia shedding light on atoms episode 7 covalent bonding page 2 of 3 7 draw an electron dot diagram of an o 2 molecule and an n. energy level periodic table The electron configuration. lewis structure for nh3 ammonia university maryland lewis structures for nh3 step by ...
I quickly take you through how to draw the Lewis Structure of Ammonia, NH3. I also go over hybridization and bond angle.

Draw an electron dot diagram for ammonia
14+ Electron Dot Structure Of Nh3. The ammonia molecule has one unshared pair of. The best way to figure this out is to draw the lewis structure. Then, using the aufbau principle, draw electron dot structures for each of the elements. This is the reason why ammonia acts as a lewis base, as it can donate those electrons. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th ... Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3.
Draw an electron dot diagram for ammonia. The lewis structure of the product that is formed. Draw the Lewis dot structure of S N F 3 , consistent with V S E P R . Draw an electron dot structure of the ethene molecule. (Without showing circle): Then we determine which is the central atom. Draw Lewis structure for one important resonance form of HNO3 HONO2. Draw the Lewis dot structure for HNO3 and provide the following information. So H 1 N 5 O 6 if we sum it up we get 153624. Sum of valence electrons from all atoms. How to Draw the Lewis Structure for NH2OH Lewis Structure. Answer the following questions about nitrogen, hydrogen, and ammonia. (a) In the boxes below, draw the complete Lewis electron-dot diagrams for N 2 and NH 3. The correct structures are shown in the boxes above. Two points are earned for the correct Lewis electron-dot diagrams (1 point each). The electron-dot structure of NH3 places one pair of nonbonding electrons in the valence shell of the nitrogen atom. This means that there are three bonded atoms and one lone pair for a coordination number of four around the nitrogen, the same as occurs in H2O. The Lewis dot structure for ammonia, NH3. Secondly, what is the shape of nh3 ...
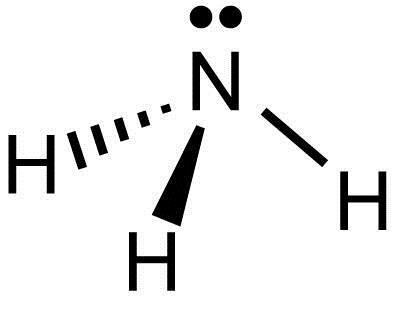
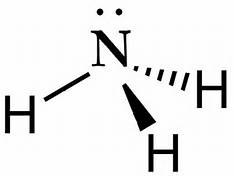
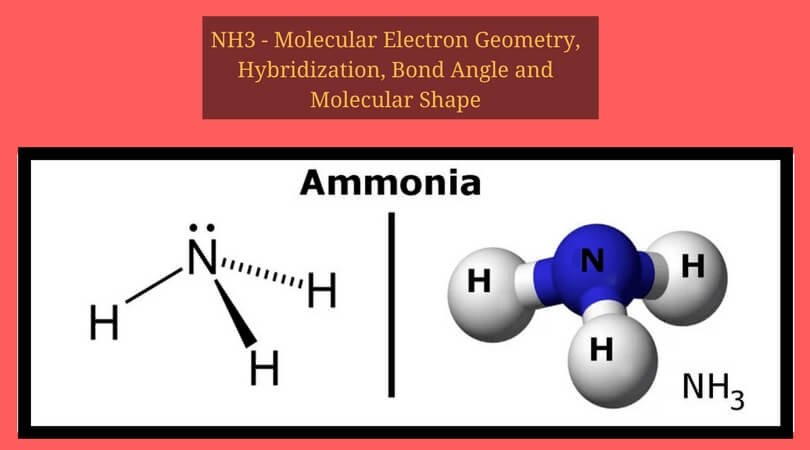
2. Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair of electrons. 3. Often the shared pair of electrons forming the covalent bond is circled. 4. Sometimes the bond itself is shown (-), these structures can be referred to as valence structures. ammonia, NH3. In this how-to video, you will learn how to make the Lewis structure for Ammonia. The formula for Ammonia is NH3. Now, write down H, N, and H in a horizontal line. Place an H under the N. Place two dots in between the spaces found in the H's and the N. Also place two dots above the N. Since the valance electrons are balanced, draw a line between the two dots connecting the H to the N. Leave ... Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride for med of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can for m an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry, and molecular geometry of this molecule. A video explanation of how to draw the Lewis Dot ... What is the Lewis dot structure for ammonia? In the lewis structure of ammonia (NH3), there are three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps. Can you draw the electron dot structure of […]
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3. Lewis Structure (electron dot diagram) for ammonia OR . Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom. Dec 10, 2016 · 1 answerThere are 8 valence electrons to distribute........... Explanation: And 3×N−H bonds, and one nitrogen-centred lone pair of electrons. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale...
Ammonia lewis structure contains three N-H bonds and one lone pair on nitrogen atom. Lewis structure of NH3 can be drawn by using valence electrons of ...
Click here to get an answer to your question ✍️ Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].Nov 19, 20191 answer · Top answer: It is as shown in the figure. Nitrogen has 5 valence electrons whereas Hydrogen has 1.
A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons.
The best way to figure this out is to draw the Lewis structure. For the NH3 Lewis structure, calculate the total number of valence electrons for the NH3 ...3 answers · 2 votes: Electrons of N is shared by H to complete its duplet and elctrons of H is shared by N to ...
Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides. We're going to do the Lewis structure for NH3: ammonia or Nitrogen trihydride. On the periodic table, Nitrogen is in group 5 or 15 ...
This is the structure of ammonia or \[N{{H}_{3}}\].-In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is shown below.
In addition to this, ammonia is considered corrosive as well as hazardous if stored in significantly larger quantities. The lewis structure that is also called an electron dot structure, is mainly a pictorial representation of the valence electrons present in an atom. The diagram is drawn using dots around the symbol of an atom, mostly in pairs.
The Lewis structure of ammonia, #NH_3#, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons on top of the atom.This is the reason why ammonia acts as a Lewis base, as it can donate those electrons.
A simple method for drawing the lewis structure ammonia. We'll first ammonium ion (nh4+). For we have an ionic compound and need take that into account when dr. A quick explanation of the molecular geometry nh3 including description bond angles. In this example, we draw the lewis structure for polyatomic cation ammonium.

By Drawing An Electron Dot Diagram Show The Formation Of Ammonium Ion Atomic No N 7 And H 1 Chemistry Shaalaa Com
Draw a electron dot diagram to show the formation of ammonium ion. Easy. Open in App. Solution. Verified by Toppr. Was this answer helpful? 0. 0. Similar questions. Draw Lewis structure for the following molecules.
The Lewis dot structure of cl2co is obtained by using the function cl2co. Lewis Dot Structure Cl2co CCl2O Lewis Structure. Well also explore polyatomic ions and how to draw Lewis dot structures for them. Alternatively a dot method can be used to draw the lewis structure of COCl 2. The dot structure for phosgene starts with the C atom in the center.
Nov 14, 2018 — Find an answer to your question draw the electron dot structure of ammonia molecule and show the formation of Ammonium Ion from meet level ...2 answers · 463 votes: plzzzzzzzzz mark my answer as brainliest
By drawing an electron dot diagram, show the lone pair effect leading to the formation of ammonium ion from ammonia gas and hydrogen ion.
Ammonia: Ammonia has the formula NH 3.It's highly soluble in water because it's a polar substance. The Lewis structure for a molecule represents the total valence electrons in the molecule.

By Drawing An Electron Dot Diagram Show The Lone Pair Effect Leading To The Formation Of Ammonium Ion Brainly In
Use information from step 4 and 5 to draw the lewis structure. Nitrogen goes in the centre. Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3. Calculate the total valence electrons in NH3 molecule. N=5,H=1x3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides.
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer.It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.. Video: Drawing the Lewis Structure for NH 3
lewis nh3 structure ammonia dot lone chemistry which electron pairs chemical forces intermolecular boiling point molecule molecular bond geometry draw
Electron Dot Structure of NH3 by Jeff Bradbury - February 17, 2012 - Lewis Electron Dot Structure for ammonia molecule NH3.
Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. and put a dot and a cross in each overlapping section (there should be three dots and three crosses in the whole diagram Actually the formula for an ammonia ion is NH4+ Due to dative bonding the 4th ...
14+ Electron Dot Structure Of Nh3. The ammonia molecule has one unshared pair of. The best way to figure this out is to draw the lewis structure. Then, using the aufbau principle, draw electron dot structures for each of the elements. This is the reason why ammonia acts as a lewis base, as it can donate those electrons.
Draw An Electron Dot Diagram To Show The Formation Of Ammonium Ion Atomic No N 7 And H 1 Sarthaks Econnect Largest Online Education Community
Draw The Electron Dot Structure Of O2 Nh3 And Ccl4 Science Carbon And Its Compounds 10050281 Meritnation Com
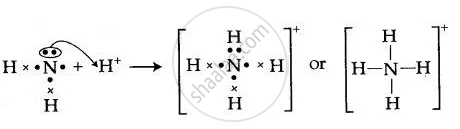
By Drawing An Electron Dot Diagram Show The Lone Pair Effect Leading To The Formation Of Ammonium Ion From Ammonia Gas And Hydrogen Ion Chemistry Shaalaa Com
By Drawing An Electron Dot Diagram Show The Lone Pair Effect Leading To The Formation Of Ammonium Ion From Ammonia Gas And Hydrogen Ion Sarthaks Econnect Largest Online Education Community




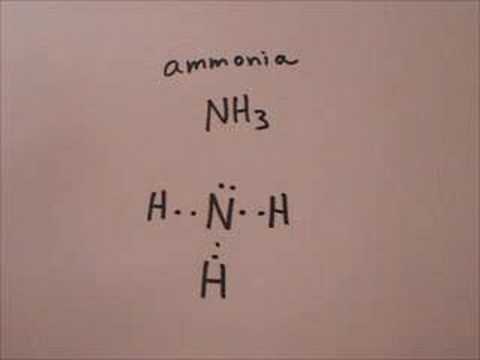
















0 Response to "37 draw an electron dot diagram for ammonia"
Post a Comment