39 energy diagram endothermic and exothermic reaction
Energy changes accompany chemical reactions. Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (E a), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Endothermic and exothermic reactions are ... Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
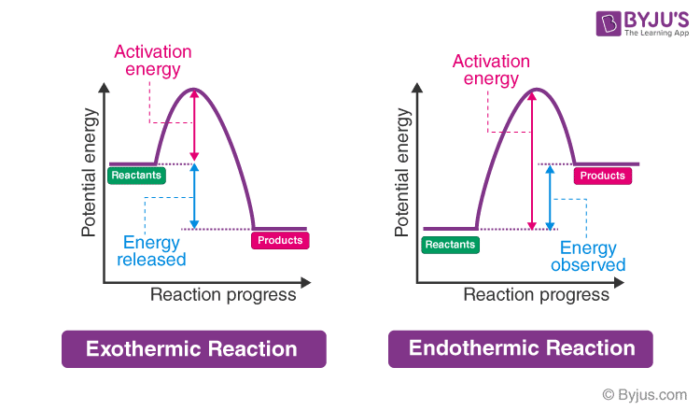
In endothermic reactions, there is less energy in the reactants than in the products. Activation Energy The activation energy in an exothermic reaction is energy at the top of the curve minus the energy of the reactants.
Energy diagram endothermic and exothermic reaction
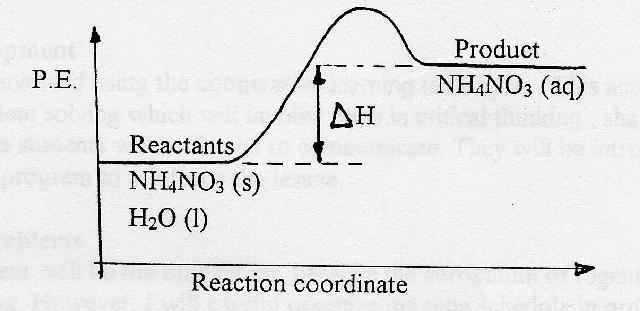
Endothermic reaction. Draw an energy coordinate diagram for both an endothermic and an exothermic reaction. In Sam's case, when ammonium nitrate was dissolved in water, the system absorbed heat from the surrounding, the flask, and thus the flask felt schematron.org is an example of an endothermic reaction. ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages Energy level diagram for an exothermic chemical reaction without showing the activation energy.It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. Could usually . has a very low activation energy.
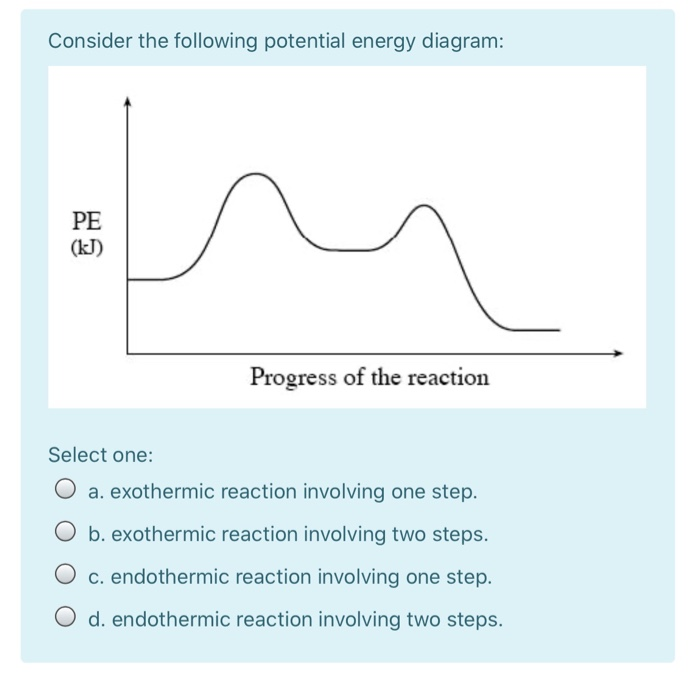
Energy diagram endothermic and exothermic reaction. Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ... Based on the following energy diagram, is the | Chegg.com. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or ... This chemistry video tutorial focuses on endothermic and exothermic reactions. It explains the flow of heat energy into and out of the ... 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
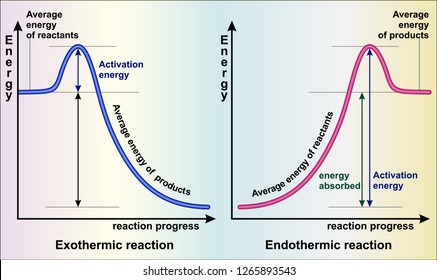
9 Jul 2019 — Figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ... a reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small? exothermic Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0). Recall that ΔH rxn = H products-H reactants. Thus if the products are lower in energy ... Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ...
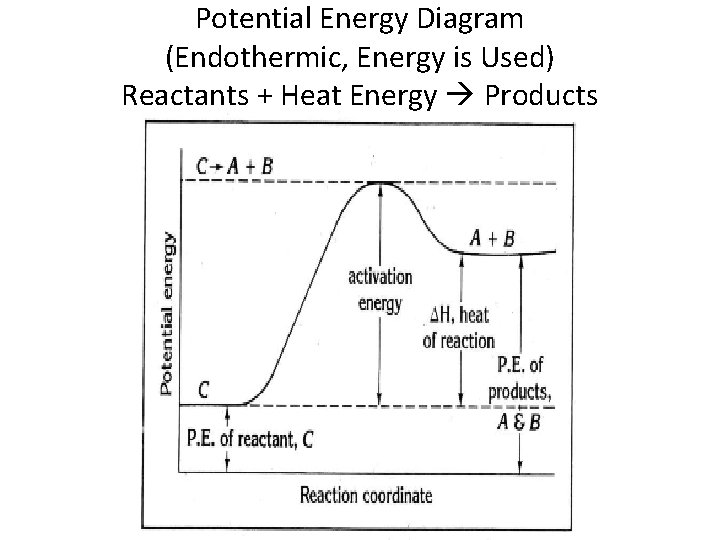
The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic - more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof . Endothermic vs. exothermic reactions. This is the currently selected item. Test prep · MCAT · Chemical processes ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer ...
Bond energy is the amount of energy absorbed to break the bonds or released energy during the formation of bonds in one mole of the substance . The breaking of bonds is an endothermic process and needs to absorb an amount of energy from the surrounding , So , its ΔH has a positive sign . The formation of bonds is an exothermic process and ...
Why is the potential energy of the reactants greater than the potential energy of the products? [9-12 Content Standard B- Conservation of energy] 5. Develop P.E. diagram for the exothermic reaction. 6. Define , = PE (Prod) - PE (React) Summary: Assign numbers to the diagram and ask students to calculate
The Course Of A Reaction Figure 13 4 Plots The Course Of A Reaction The Initial Average Energy Of The Reactants Is Indicated At The Left Side Of Each Graph If Molecules Are To Collide Effectively They Must Have More Than The Average Energy They Must Have
Endothermic and exothermic reactions are chemical reactions that absorb and release heat, respectively. A good example of an endothermic reaction is photosynthesis. Combustion is an example of an exothermic reaction. The categorization of a reaction as endo- or exothermic depends on the net heat transfer. In any given reaction, heat is both ...

Question Video Identifying Steps In A Reaction Profile Diagram For A Two Step Chemical Reaction Nagwa
Reaction energy diagrams show how the energy of a system changes as the reactants form the products. On the diagram, we can label the activation energy and the ...1 answer · Top answer: Definitions Activation Energy (EAA) - the activation energy is the minimum amount of energy the reactants need in order for the reaction...
Task 2: Introduction to Energy Diagrams Click here to fill help you fill in this task: Endothermic vs. Exothermic and Energy Diagrams Reference Slides Energy Diagram Shows the changes in potential energy during a reaction. It starts with the _reactants_____ on the left and proceeds to the ____products_____ on the right.
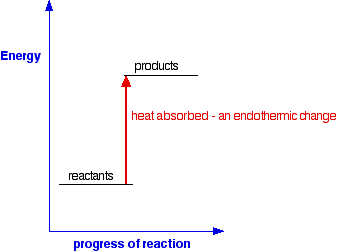
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Exothermic reactions release energy to their surroundings, because the products are lower in energy than the reactants. You can think about this visually using a reaction energy diagram, as seen below: And endothermic reaction (left) and an exothermic reaction (right) plotted on a plot of energy against the reaction coordinate (a measure of the ...
An energy level diagram for an exothermic reaction In an endothermic reaction, the products are at a higher energy than the reactants. This means that the enthalpy change of the reaction (∆ H ...
What Is Difference Between Endothermic And Exothermic Reaction If Both Require Activation Energy Quora
How does the energy level diagram show this reaction is exothermic? Energy profile diagrams for endothermic and exothermic reactions. Every chemical substance has a certain amount of chemical energy. This energy is given the symbol H and is different for different substances. It is difficult to measure the absolute energy of a substance but the change in energy during chemical reactions can be ...
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...

Exothermic Energy Diagram Activation Energy Transition States And Enthalpy Change Tutor Hotline Youtube
c) Draw a labeled enthalpy level diagram for an exothermic and endothermic reaction showing the activation energy, Ea and enthalpy change. [4] 9. (M05/S/2) In a neutralization reaction 50 cm 3 of a 0.50 moldm-3 solution of sodium hydroxide is mixed rapidly in a glass beaker with 50 cm 3 of a 0.050 moldm-3 solution of sulfuric acid.
A reaction that takes in heat energy so the temperature goes down on a energy profile diagram is it a exothermic or endothermic reaction if the activation energy is small. In the case of an endothermic reaction the reactants are at a lower energy level compared to the productsas shown in the energy diagram below.
What Is The Difference Between An Exothermic Reaction And Endothermic In Which Case We Use The Catalyst
The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more.
Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).
Exothermic and endothermic reactions. When a chemical reaction occurs, energy is transferred to or from the surroundings. There is usually a temperature change. For example, when a bonfire burns ...
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg.
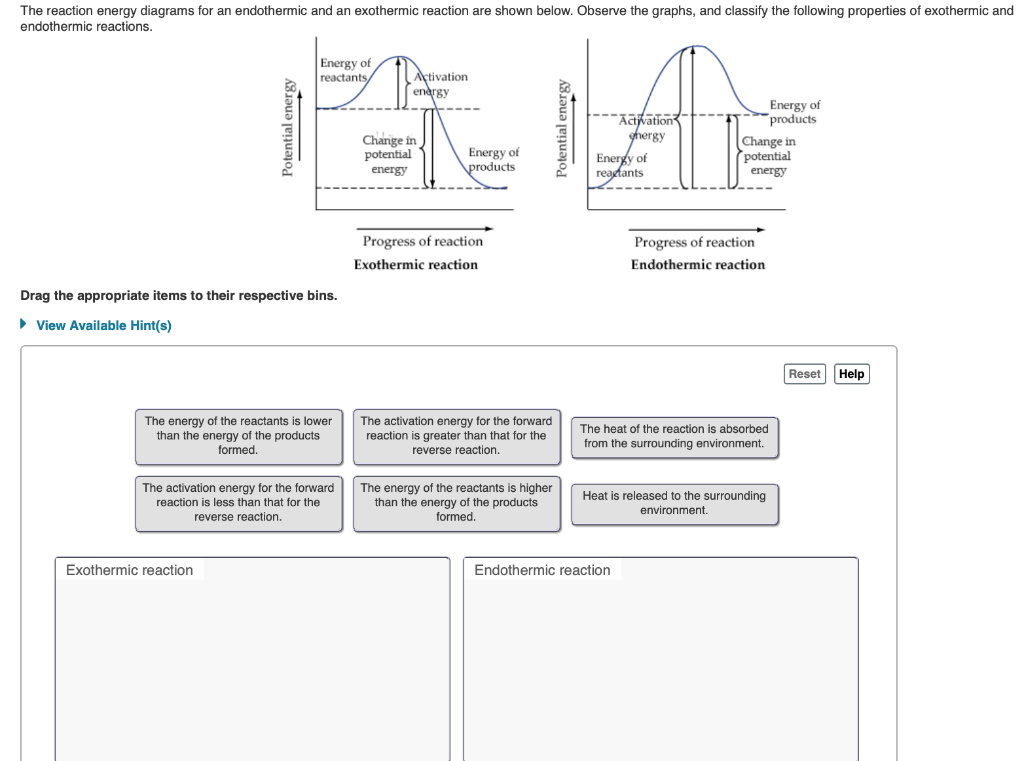
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ...
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
Energy Profile for Exothermic Reactions. The synthesis of ammonia gas (NH 3 (g)) from nitrogen gas (N 2 (g)) and hydrogen gas (H 2 (g)) is an exothermic reaction. 92.4 kJ mol -1 (of N 2 (g)) is released. Energy (heat) is a product of the reaction: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1. In order for energy to be conserved during the ...
Energy level diagram for an exothermic chemical reaction without showing the activation energy.It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy. Could usually . has a very low activation energy.
ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages
Endothermic reaction. Draw an energy coordinate diagram for both an endothermic and an exothermic reaction. In Sam's case, when ammonium nitrate was dissolved in water, the system absorbed heat from the surrounding, the flask, and thus the flask felt schematron.org is an example of an endothermic reaction.

Chemistry Alert Endothermic And Exothermic Reactions Energy May Also Be Absorbed Or Released In A Reaction When More Energy Is Released Than Absorbed The Reaction Is Said To Be Exothermic When


















0 Response to "39 energy diagram endothermic and exothermic reaction"
Post a Comment