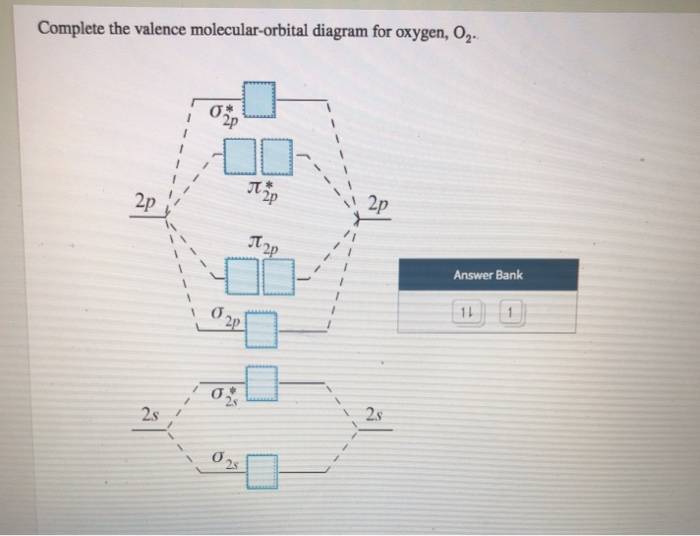
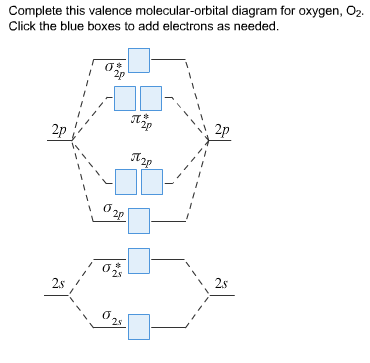
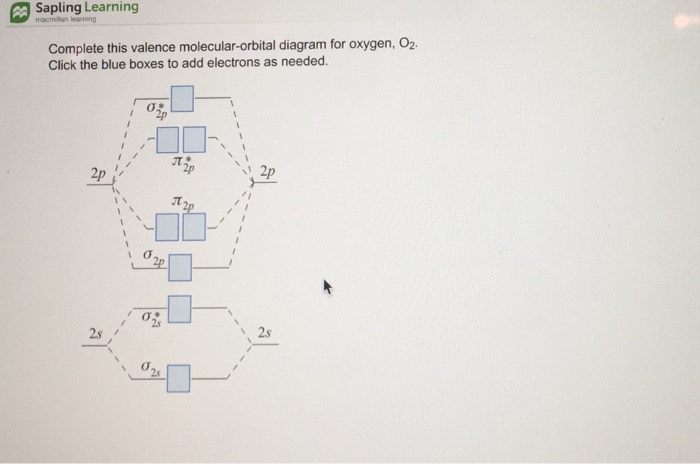
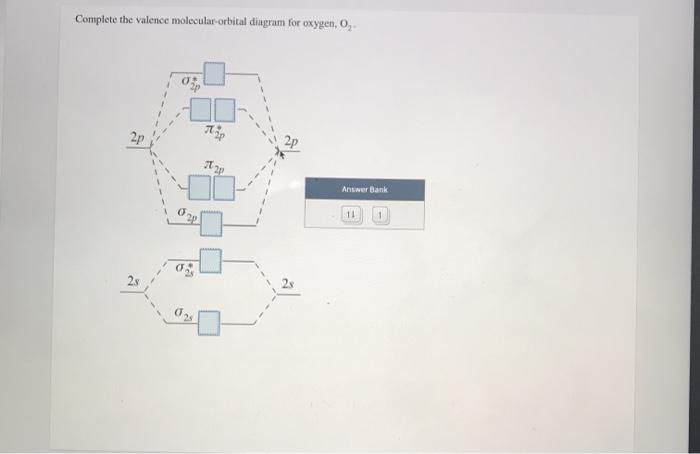
40 complete this valence molecular-orbital diagram for oxygen o2
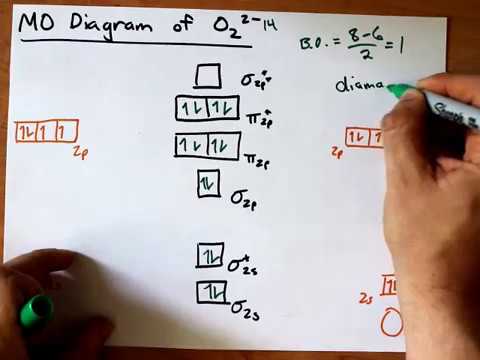
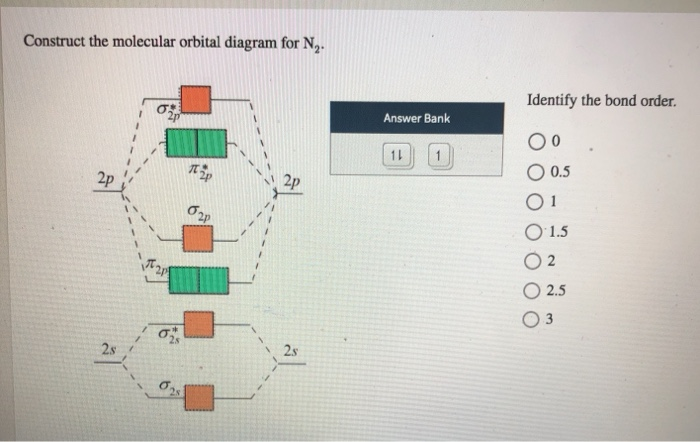
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
The electrons are arranged in shells around the nucleus. e N3- has 7+3 = 10 electrons O2- has 8+2= 10 electrons F- has 9+1 = 10 electrons Na+ has 11-1 = 10 electrons Mg2+ has 12-2 = 10 electrons Al3+ has 13-3= 10 electrons Manipal 2005: Which among the following species have the same number of electrons in its outermost as well as penultimate shell? (A) Mg2+ (B) O2- Sep 12, 2010 · It could be ...
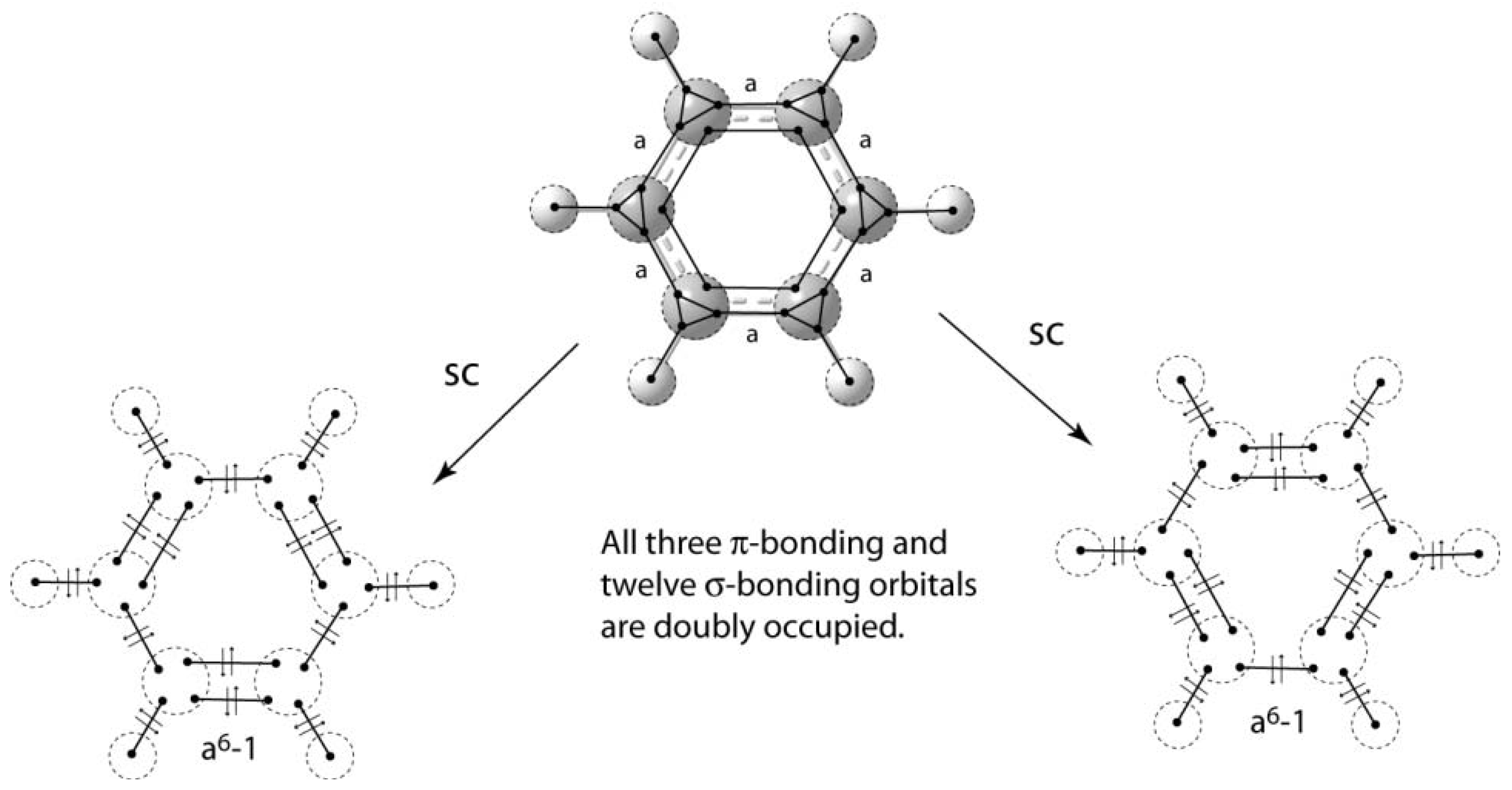
Complete this valence molecular-orbital diagram for oxygen o2
The first compound in the diagram has two blue and three red atoms. The second compound has two blue and ... O2, oxygen I2, iodine. F2, fluorine. Magnesium reacts with oxygen to give (yield) magnesium oxide. The reactants are Mg and O2. The product is MgO. Review Problems 1.30. 3 Fe, 2 Al, 3 Si, 12 O. 1.31. 3 Ca, 5 Mg, 8 Si, 24 O, 2 H. 1.32 . KNaC4H4O6. 1.33. MgSO47H2O Chapter 1. 1.34. CH3COOH ... Oxygen has '6' valence electrons. ” [See separate tutorial for determining formula charges. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. docx from CHEM 161 at Bellevue College. You may notice from Figure 4. Formal Charge –charge assigned to each atom in a ... 1.71 (a) 3.41 _ 0.23 3.18 x 0.205 = x 0.205 = 0.12457 = 0.125 5.233 5.233 Complete the subtraction first. The result has 2 decimal places and 3 significant figures. The result of the multiplication and division must have 3 significant figures. Since the digit to be dropped is 5 with nonzero digits following, round up. (b) 5.556 x 2.3 5.556 x 2.3 = = 3.08 = 3.1 4.223 _ 0.08 4.143 Complete the ...
Complete this valence molecular-orbital diagram for oxygen o2. NCERT Exemplar Solutions Class 11 Chemistry Chapter 4 – Free PDF Download. NCERT Exemplar Chemistry Class 11 Chapter 4 Chemical Bonding and Molecular Structure is provided here to help students develop a clear approach to chemical bonding. Chemical Bonding and Molecular Structure is a very important topic that lays the foundation for all your future studies. FREE Answer to Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on molecular orbital diagram. A molecular orbital diagram is used to explain ... Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. The last two electrons in p 2 p x ∙ and p 2 p y ∙ orbitals will remain unpaired. Therefore, oxygen molecule has paramagnetic character due to the presence of two unpaired electrons. Complete this valence molecular orbital diagram for oxygen o2. Click the blue boxes to add electrons as needed. Complete these structures by adding bonds and lone pairs as necessary. This video shows the construction of a molecular orbital mo diagram for the diatomic molecule o2 using the valence electrons of each oxygen.
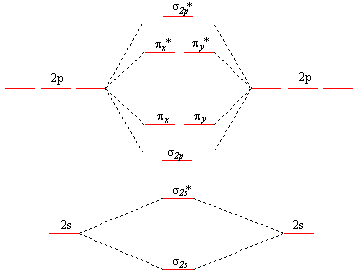
oxygen being the limiting reagent. 100 g CH4 × (1 mol / 16 g) = 6.25 mol 100 g O2 × (1 mol / 32 g) = 3.125 mol In addition to having fewer moles of oxygen, 2 moles of oxygen are required to react with 1 mole of methane; oxygen is definitely the limiting reagent. From this, we can be certain that 0 moles of oxygen will remain Academia.edu is a platform for academics to share research papers. Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Academia.edu is a platform for academics to share research papers. According to molecular orbital theory, what is the bond order in the O2+ ion? Select one: a. 5.5 b. 5 c. 4 d. 2.5 e. 1.5. The correct answer is: 2.5. Valence bond theory predicts that iodine will use _____ hybrid orbitals in ICl2-. Select one: a. sp2 b. sp3 c. sp3d d. sp3d2 e. None of these choices is correct. The correct answer is: sp3d. Which is the most reasonable prediction for the H-C-H ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we’ll see that symmetry will help us treat larger molecules in

Symmetry Free Full Text Chemical Reasoning Based On An Invariance Property Bond And Lone Pair Pictures In Quantum Structural Formulas Html
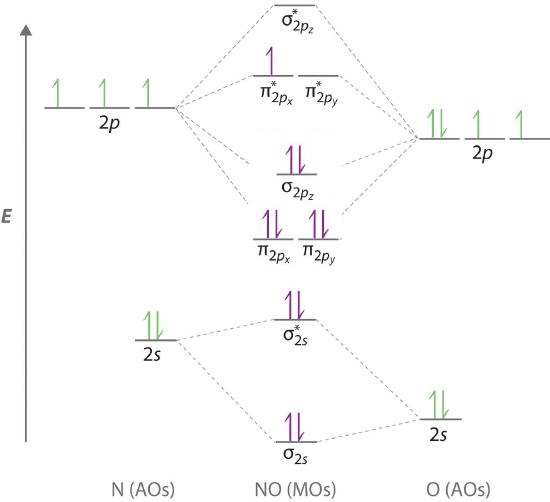
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen.
The normal valence of the C is 4 but the normal valence and the normal valence of O is 2. Covalent bonds can be formed with one or more pairs of electrons. 50mm thick and has a 2. oxygen B. S D. solid b. Based on the number of shared electron pairs, there are three types of covalent bonds [1-6]: 1. Tie up loose ends. Electrons are shared equally between the and atoms. Just as energy is always ...
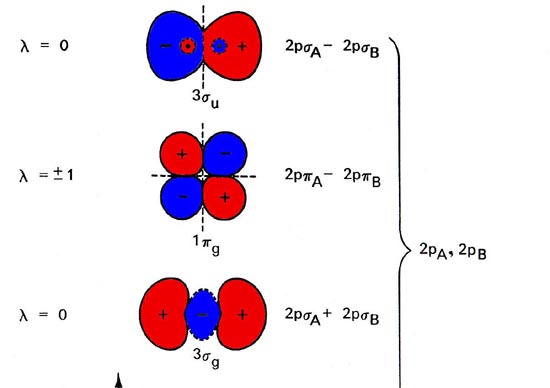
In order to draw oxygen's molecular orbital diagram, you need to start by taking a look at what atomic orbitals you have for an oxygen atom, #"O"#.. As you know, oxygen is located in period 2, group 16 of the periodic table and has an atomic number equal to #8#.This means that the electron configuration of a neutral oxygen atom must account for #8# electrons.
1.71 (a) 3.41 _ 0.23 3.18 x 0.205 = x 0.205 = 0.12457 = 0.125 5.233 5.233 Complete the subtraction first. The result has 2 decimal places and 3 significant figures. The result of the multiplication and division must have 3 significant figures. Since the digit to be dropped is 5 with nonzero digits following, round up. (b) 5.556 x 2.3 5.556 x 2.3 = = 3.08 = 3.1 4.223 _ 0.08 4.143 Complete the ...
Oxygen has '6' valence electrons. ” [See separate tutorial for determining formula charges. Total the count and then consider the formal charge on the central atom since the oxidation state for the central atom plus the groups attached must equal the atoms formal charge. docx from CHEM 161 at Bellevue College. You may notice from Figure 4. Formal Charge –charge assigned to each atom in a ...
The first compound in the diagram has two blue and three red atoms. The second compound has two blue and ... O2, oxygen I2, iodine. F2, fluorine. Magnesium reacts with oxygen to give (yield) magnesium oxide. The reactants are Mg and O2. The product is MgO. Review Problems 1.30. 3 Fe, 2 Al, 3 Si, 12 O. 1.31. 3 Ca, 5 Mg, 8 Si, 24 O, 2 H. 1.32 . KNaC4H4O6. 1.33. MgSO47H2O Chapter 1. 1.34. CH3COOH ...

Complete This Molecular Orbital Diagram For Cn Then Determine The Bond Order Note That The 1s Homeworklib

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are

Complete This Valence Molecular Orbital Diagram For Oxygen O2 Click The Blue Boxes To Add Electrons As Homeworklib

Lewis Structure Oxygen Electron Shell Molecular Orbital Diagram Germanium Chemical Element Text Electron Png Pngwing

















0 Response to "40 complete this valence molecular-orbital diagram for oxygen o2"
Post a Comment