40 lewis dot diagram hcn
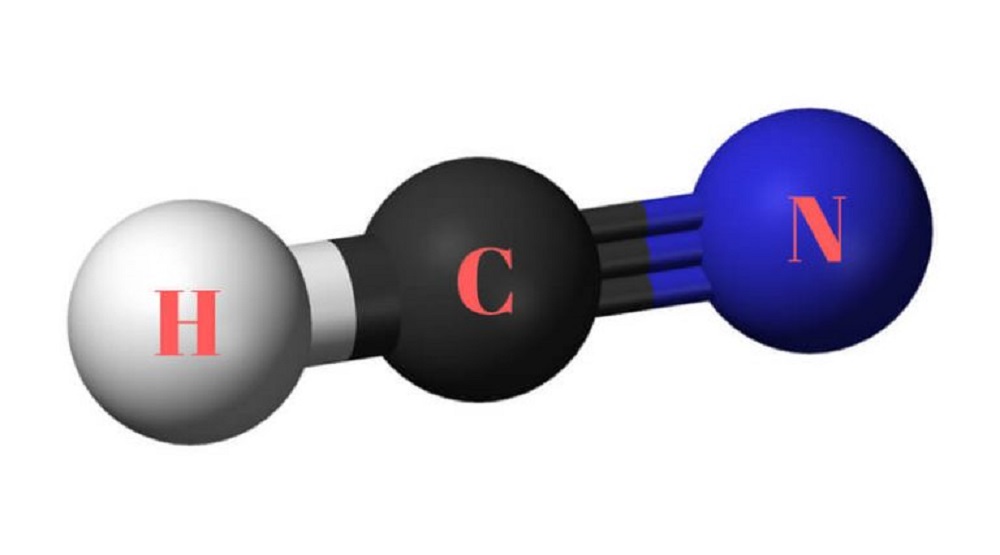
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. The lewis structure lewis dot diagram for hcn. Fill outer atoms with electrons 5. First of all refer to the periodic table and count the electrons by matching the columns. It is an ionic compound so it would not have a lewis dot structure. So the answer would be cu and a. Lewis dot structures can be drawn for a variety of molecules based on a ...
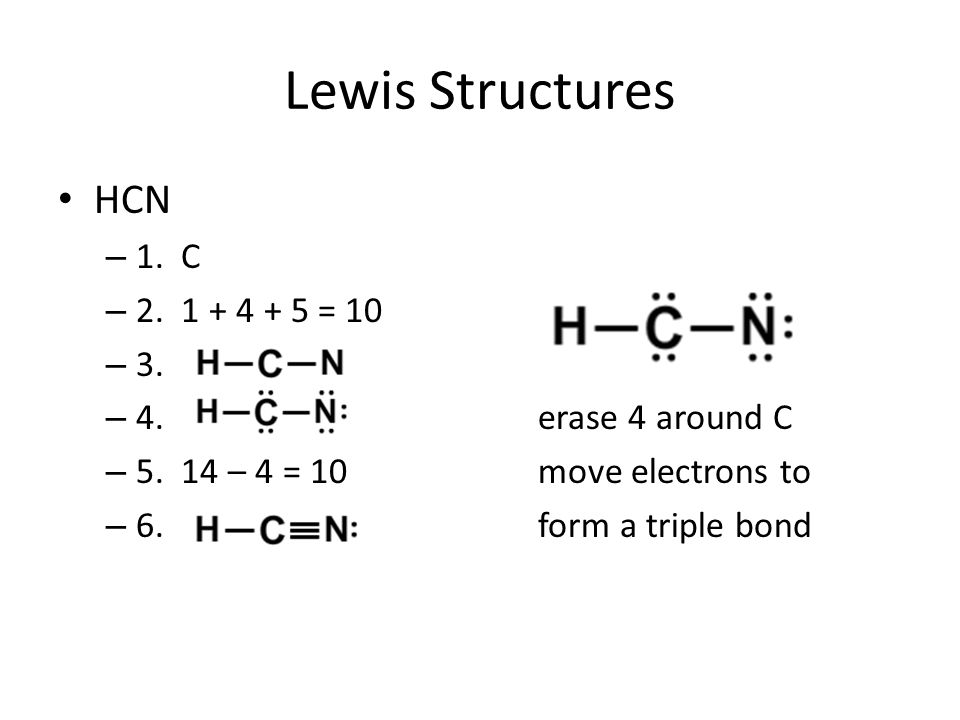
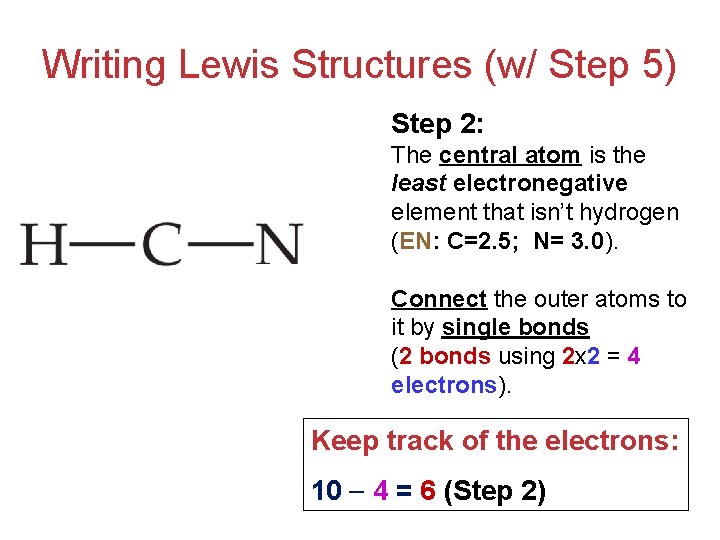
For the HCN Lewis structure, calculate the total number of valence electrons for the HCN molecule. After determining how many valence electrons there are ...

Lewis dot diagram hcn
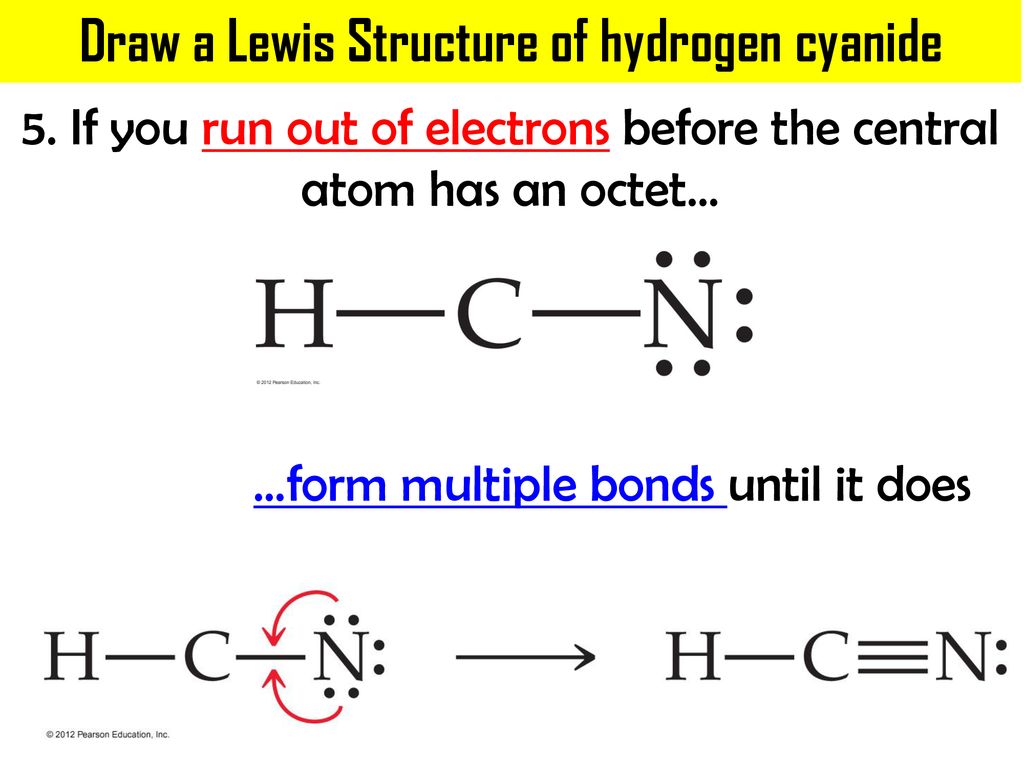
Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule. Drawing the Lewis Structure for HCN. Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you'll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available. If playback doesn't begin shortly ... Skletal structure for HCN is HCN Step III. Place one shared pair od electrons between both the pairs of atoms and show the remaining six ...
Lewis dot diagram hcn. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion. Using the Periodic Table to Draw Lewis Dot Structures. The periodic table has all of the information needed to draw a Lewis dot structure. Each Group, or column, is indicated by a roman numeral which represents the number of valence electrons. The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out... Answer to: Draw the Lewis structure for HCN. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can also... Alternatively a dot method can be used to draw the hcn lewis structure. Source: upload.wikimedia.org A simple procedure for writing lewis electron dot structures is given in a previous article entitled lewis structures and the octet rule.
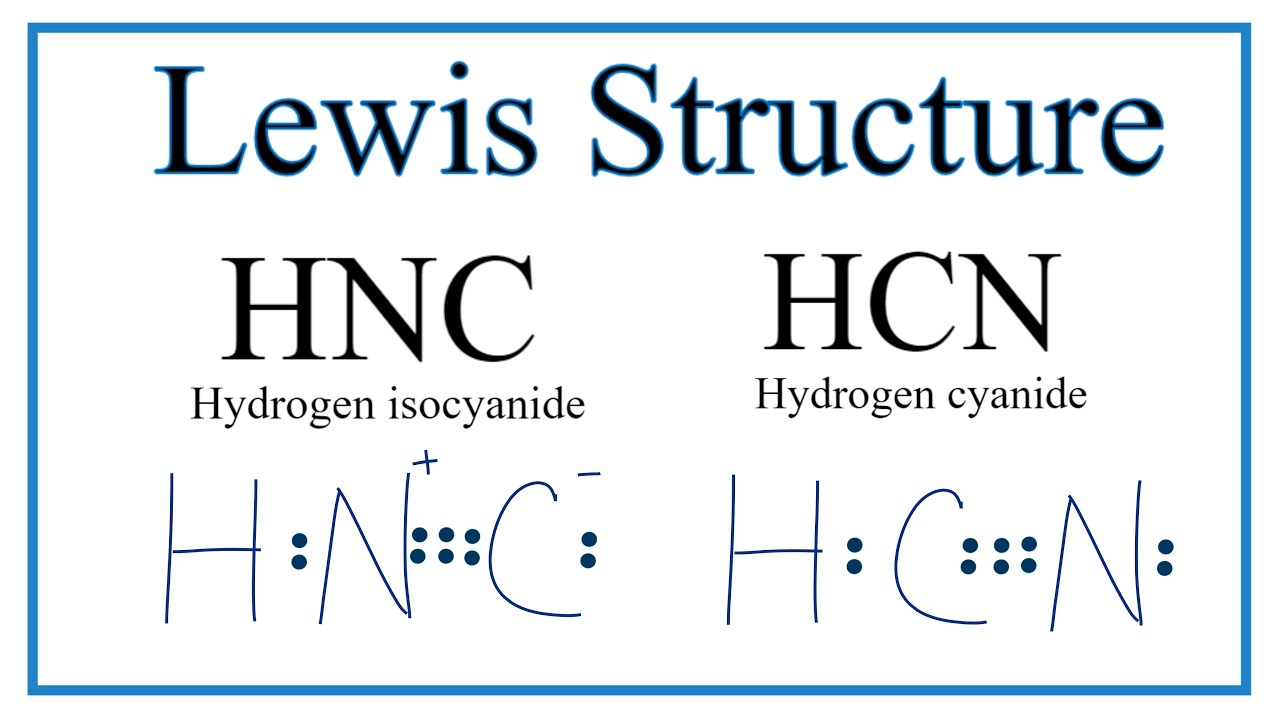
Hydrogen Cyanide (HCN) is a colorless, flammable, and poisonous liquid. HCN Lewis structure comprises three different atoms: Hydrogen, carbon, and nitrogen. It is a polar molecule with bond angles of 180 degrees. HCN is used in electroplating, mining, and as a precursor for several compounds. Name of molecule. • (3c) ·Lewis dot diagrams are used to represent valence electrons in an element. Structural formulas show the arrangements of atoms and bonds in a molecule and are represented by Lewis dot structures. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding. The bonding between the different atoms in covalent molecules is shown by some diagrams known as the Lewis structures. These also show the presence of lone pairs in the molecule. These are also known as Lewis dot diagrams, electron dot diagrams, Lewis dot structures or Lewis dot formula. The Lewis structure of hydrogen cyanide is shown below. This is a linear compound due to the presence of a triple bond between carbon and nitrogen...
HCN, which is described by the chemical formula, is one of those molecules with a unique Lewis structure. Electroplating, refining, and as a base for other substances all use this liquid. Complete answer: It is essential to know the total number of valence electrons in any molecule before drawing the Lewis dot structure. Draw a Lewis dot structure for each molecule. CF 4 CH 4 CH 3 F CH 2 F 2 C F F F F C H H H H C H H H F C H F H F *F in any position *F in any position. Cl Cl Lewis structure Worksheet-Answer Key H 2 O NH 3 PCl 3 H 2 S H 2 F 2 H O H N H H H Dr. Scott Beaver Page 2 of 3 Cl P H S H H H F F. O 2 N 2 CO 2 CS 2 CH 2 O HCN N N Lewis structure Worksheet ... See Answer. Best Answer. Copy. Please click on the related link below to see an image of the Lewis structure of HCN. H:C:::N: Wiki User. ∙ 2012-06-03 21:58:06. This answer is: Helpful. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. A step-by-step explanation of how to draw the Li2S Lewis Dot Structure. The formula is HCN.
HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds.
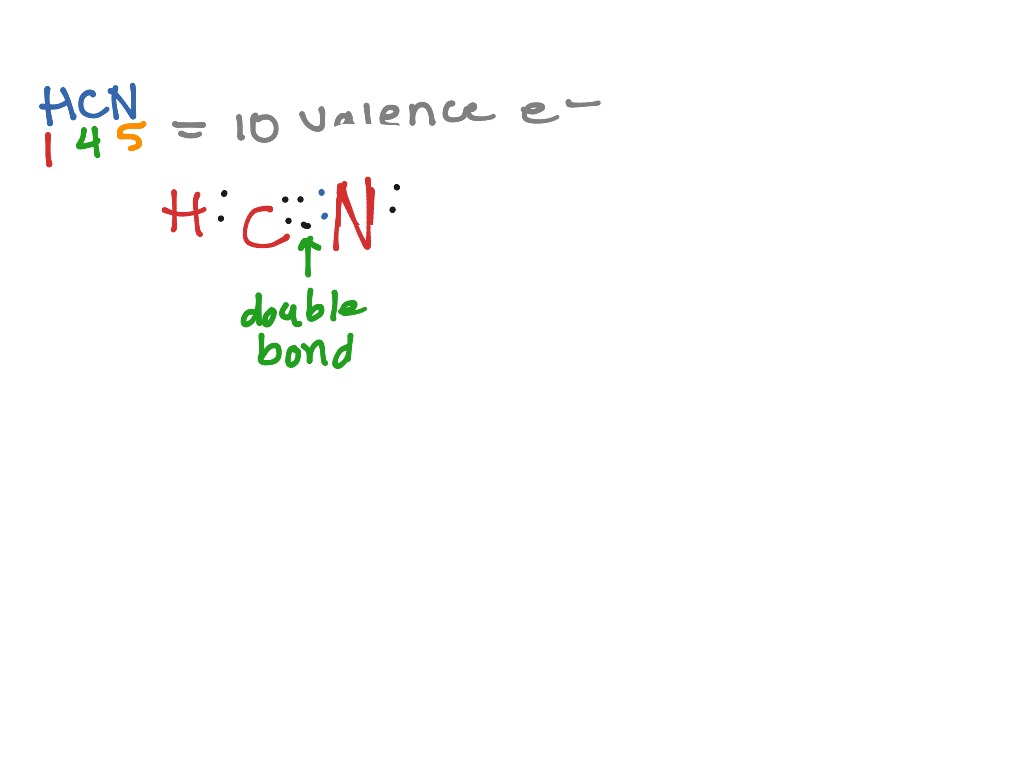
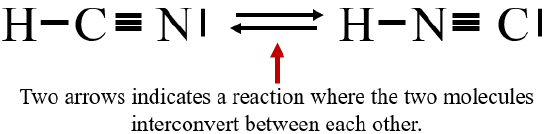
The lewis structure lewis dot diagram for hcn. Whats the combination for hcn. Draw the skeletal structure showing how the atoms are connected using single bonds. Hcn lewis structure is h single bond c triple bond n. Youre a selfie and a skin quiz away from clear healthy skin. This is how lewis dot structure of hydrogen cyanide goes.
HCN has a linear geometry but unlike nonpolar CO2 is polar due to the intrinsic electronegativity on nitrogen and the stronger force by ...
H:C:::N:The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond the carbon atom to ...
Count the valence.So that is the Lewis dot structure. What is the Lewis dot structure for XeF2? Xenon (Xe) does not have to follow the octet rule because of itsaccess to the 4d sublevel. The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a nitrogen atom.
Method 1: The easiest to teach and understand is have you memorize a list of elements. That list is; C, Si, N, P, S, O. These elements are also in priority order. So if you have covalent compound with C and P then C is the central atom because it comes first on the list. This list will cover your needs on about 95% of questions you encounter ...
Lewis dot structure of HCN. Alternatively a dot method can be used to draw the lewis structure of BF 3. Calculate the total valence electrons in BF 3 molecule. H:1 C:4 N Total= A Lewis structure is a graphic representation of the electron distribution around atoms. The reason for learning to draw Lewis structures is to predict the number .HCN ...
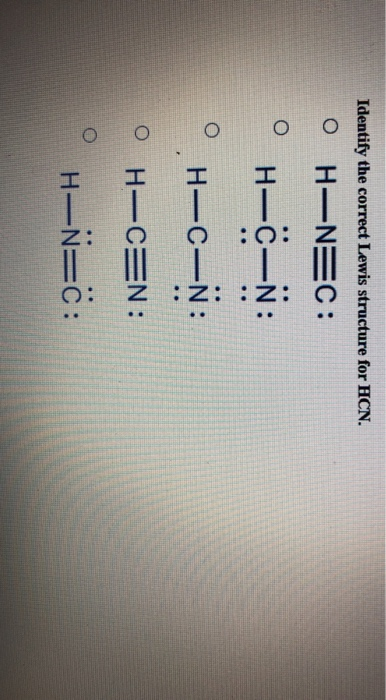
Solved Draw Charge Minimized Lewis Structures For The Following Compounds 2 Marks Each A Hcn B Course Hero
Lewis dot diagrams for elements are a handy way of picturing valence electrons, and especially, what electrons are available to be shared in covalent bonds. The valence electrons are written as dots surrounding the symbol for the element: one dot is place on each side first, and when all four positions are filled, the remaining dots are paired ...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.

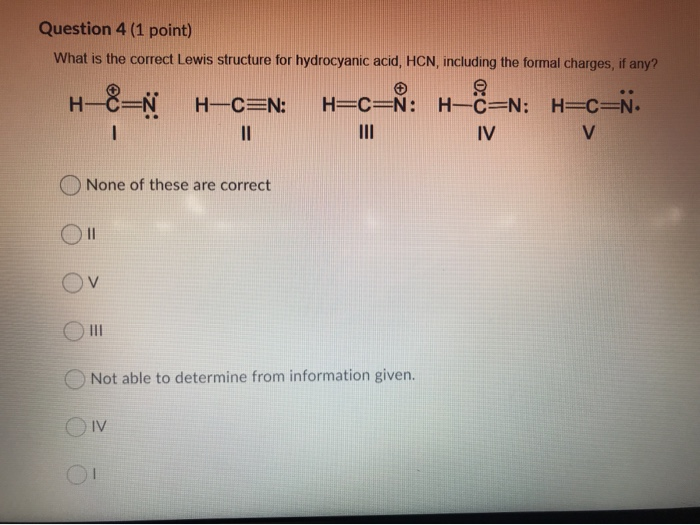
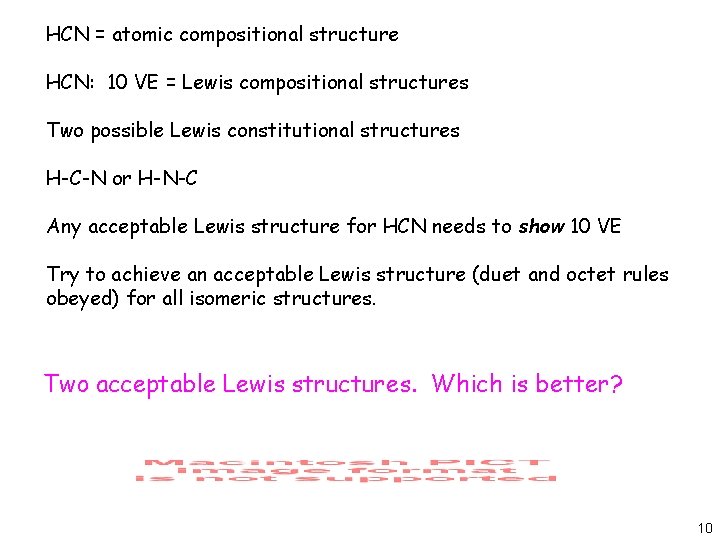
Two Possible Lewis Structures For The Molecule Hcn Are Given Determine The Formal Charge On Each Atom In Both Structures Study Com
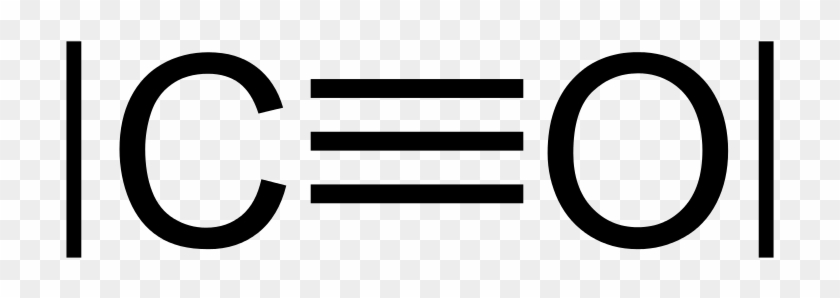
Cyanide anion, 5 + 4 valence electrons: [:C-=N:]^-. There is a formal negative charge associated with this anion. Where does it reside? The nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; the associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. The carbon atom has (or shares ...

37 Lewis Dot Structure Of Hcn How To Draw Lewis Structures Class 11 Chemistry Chemical Bonding Youtube
Lewis Electron Dot Structure With Answer Key - Displaying top 8 worksheets found for this concept.. Some of the worksheets for this concept are Lewis dot structures and molecule geometries work, Lewis structures practice work, Work 14, Chemical bonds lewis dot structures work, Practice problems h s so ch br hcn, Lewis structure work, , Ws lewis ...
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Lewis structure basically tells us how the electrons are paired. In this every dot represents an electron and pair of dots between chemical symbols for atoms represents the bond. The main steps of draw a Lewis structure are as follows: 1.
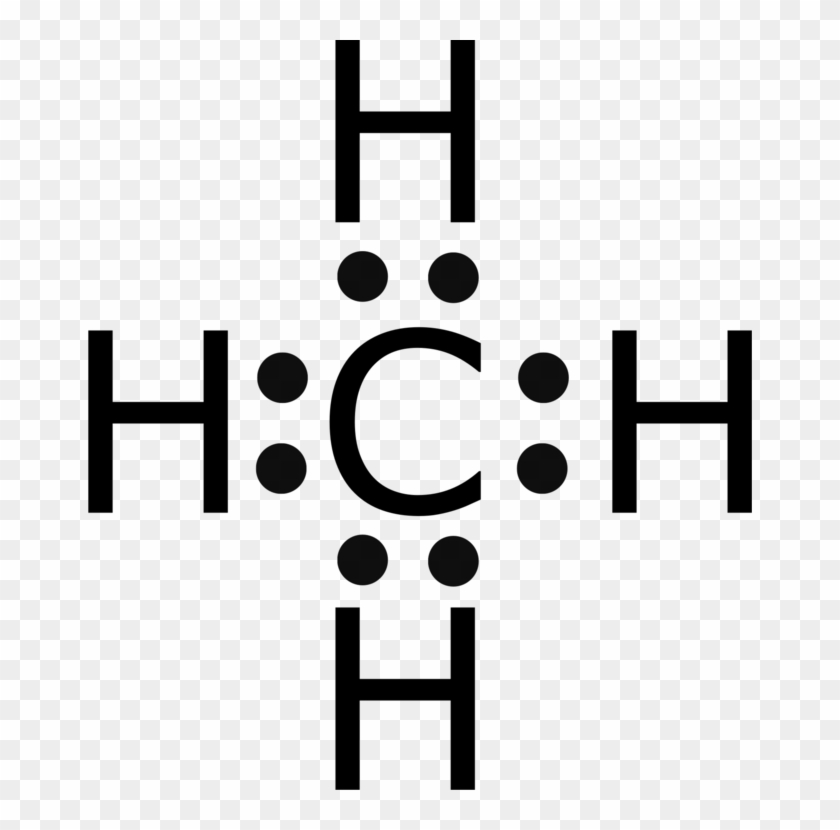
Lewis Structure Methane Electron Atom Hydrogen Lewis Dot Diagram Of Methane Free Transparent Png Clipart Images Download
Skletal structure for HCN is HCN Step III. Place one shared pair od electrons between both the pairs of atoms and show the remaining six ...
Drawing the Lewis Structure for HCN. Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you'll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available. If playback doesn't begin shortly ...
Lewis dot structure of H 2 CO. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. H:1x2=2 C:4 O Total= May 03, · May 6, - Uploaded by Wayne Breslyn. For the CH2O Lewis structure, calculate the total number of valence electrons for the CH2O molecule.















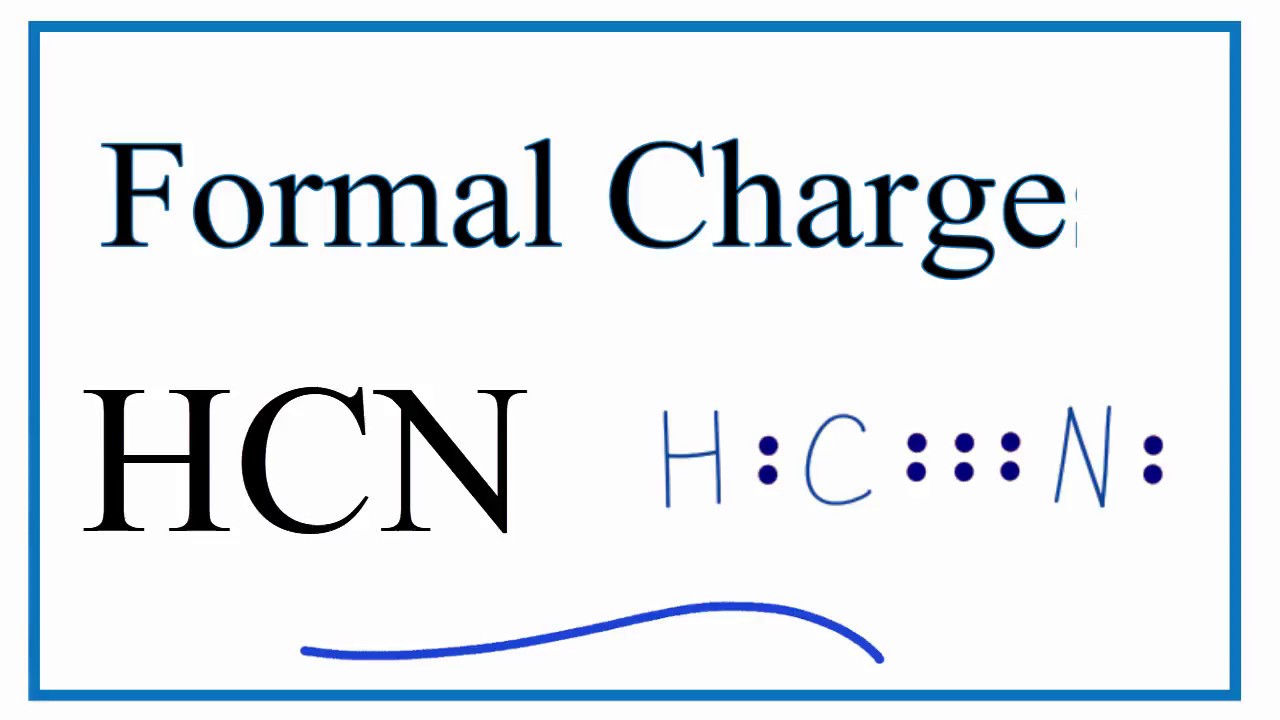





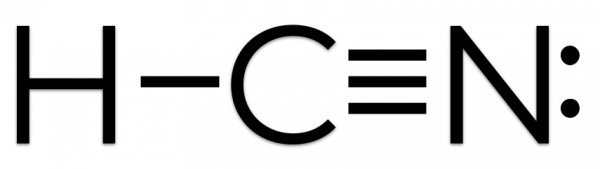
0 Response to "40 lewis dot diagram hcn"
Post a Comment