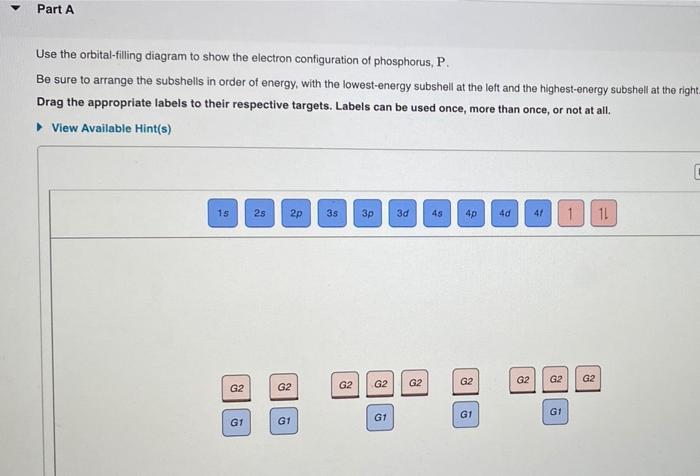
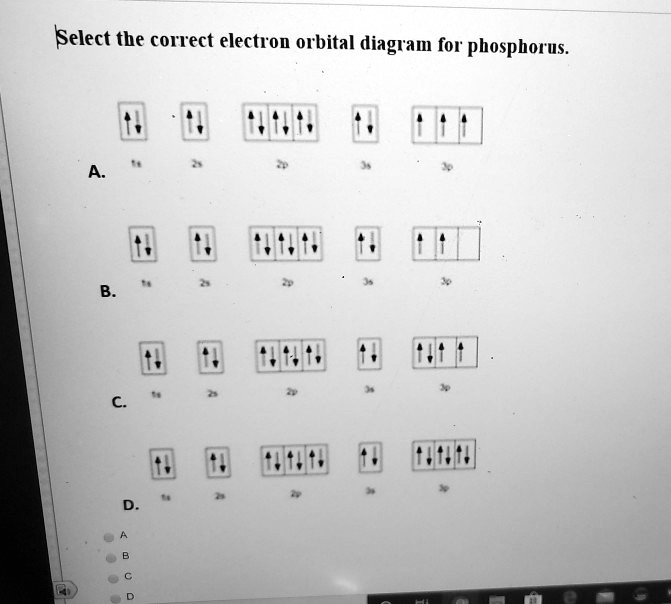
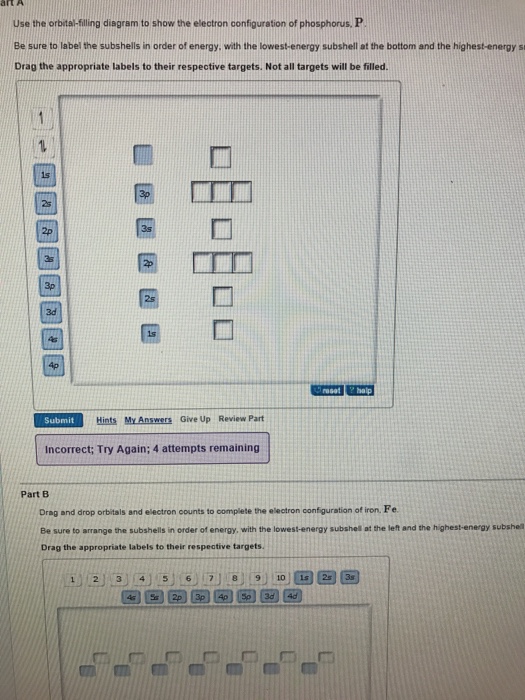
40 use the orbital-filling diagram to show the electron configuration of phosphorus, p.
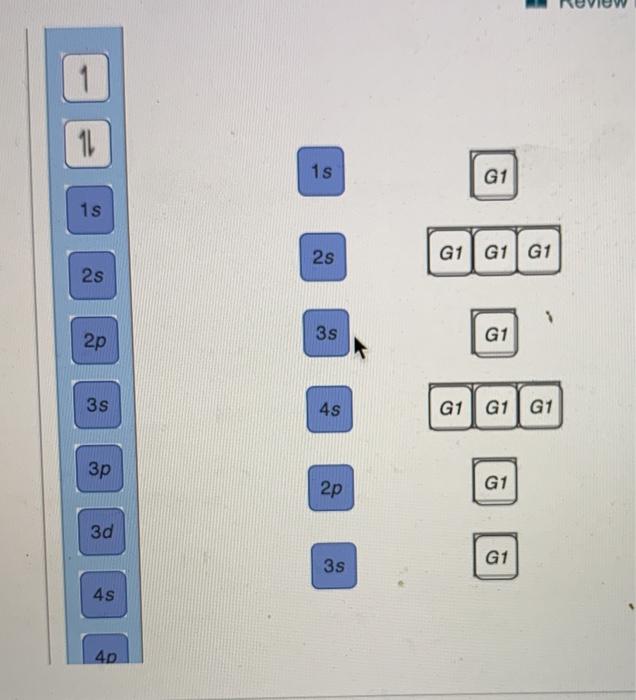
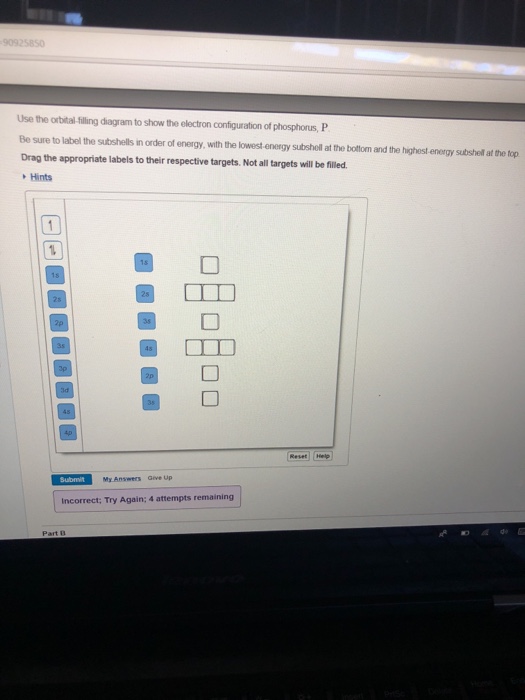
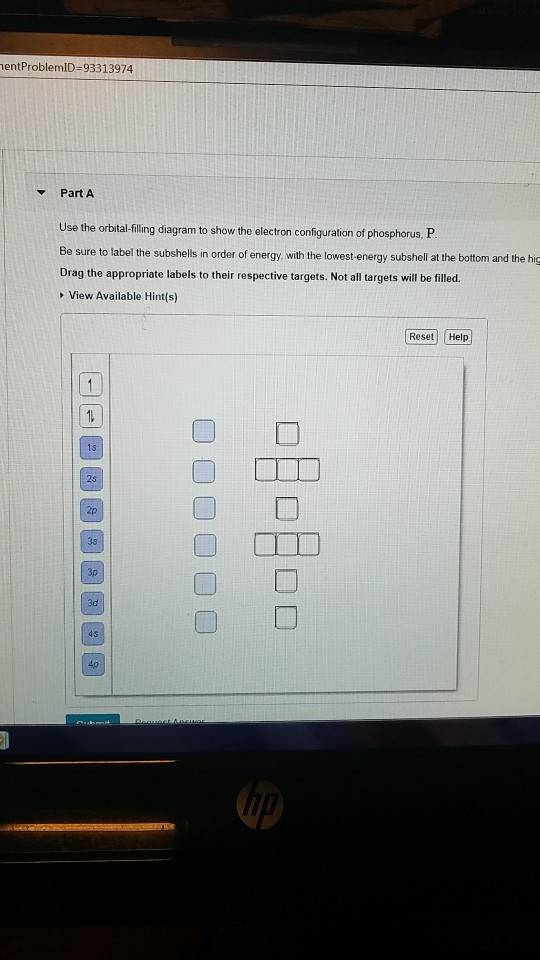
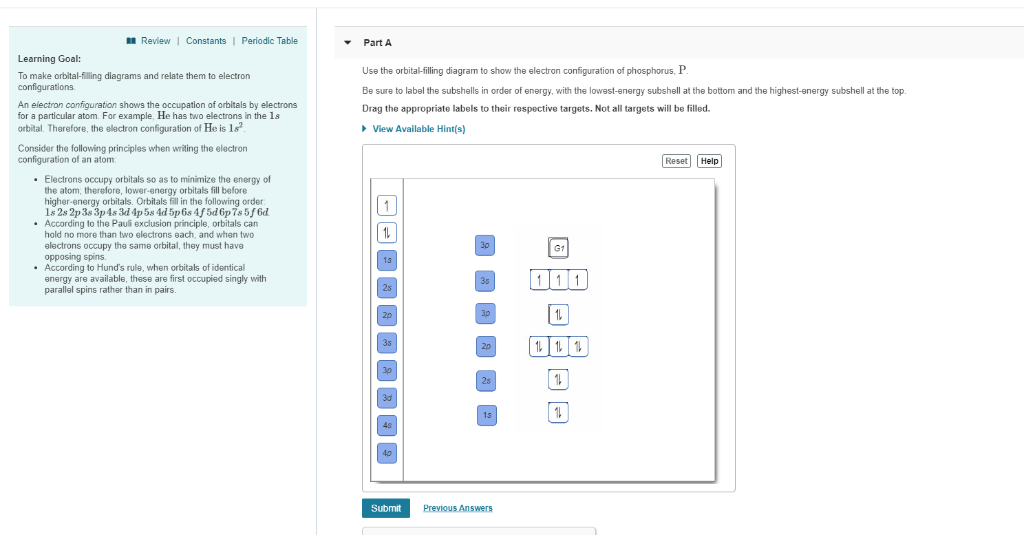
Part A Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to arrange the subshells in order of energy, with the lowest-energy subshell at the left and the highest-energy subshell at the right Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Electron Orbital Filling Rules. ... We can use the electron energy filling diagram to complete an example of how electrons in an element are arranged within the electron orbitals (Figure 2.13). ... In the analysis of the electron configuration of phosphorus, we can see that the 3rd shell is …
Valence electrons worksheet

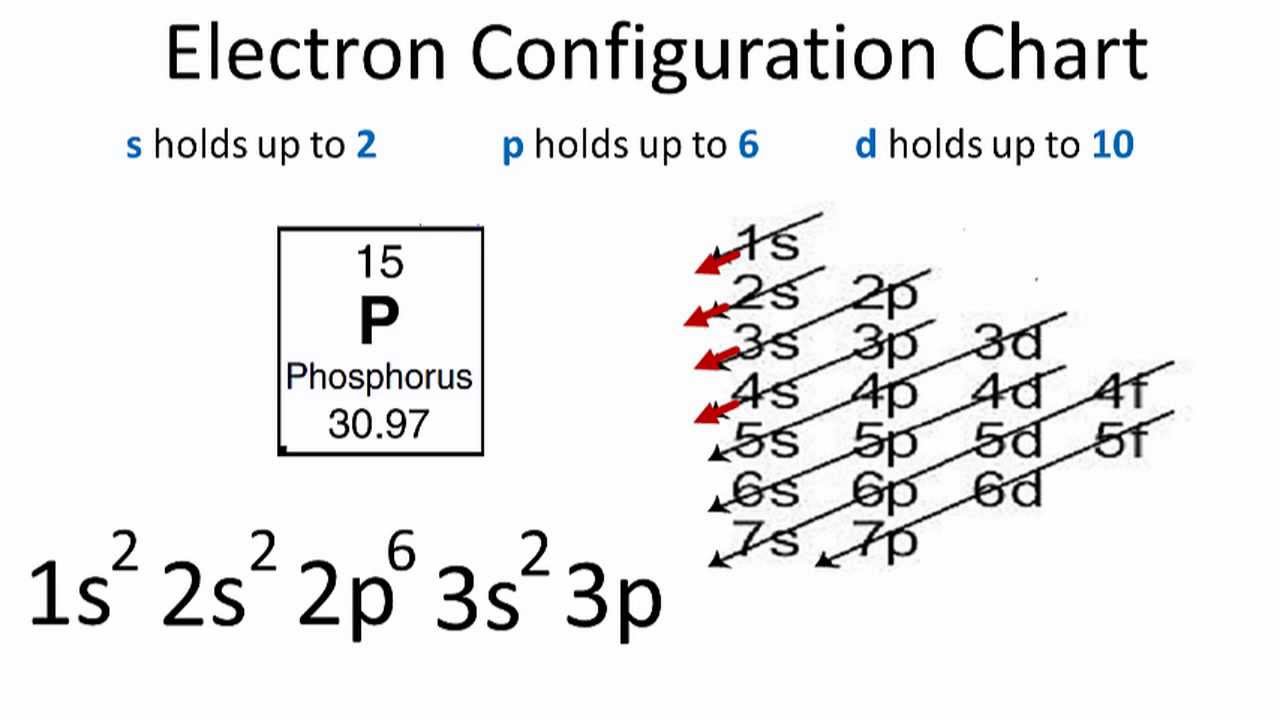
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.
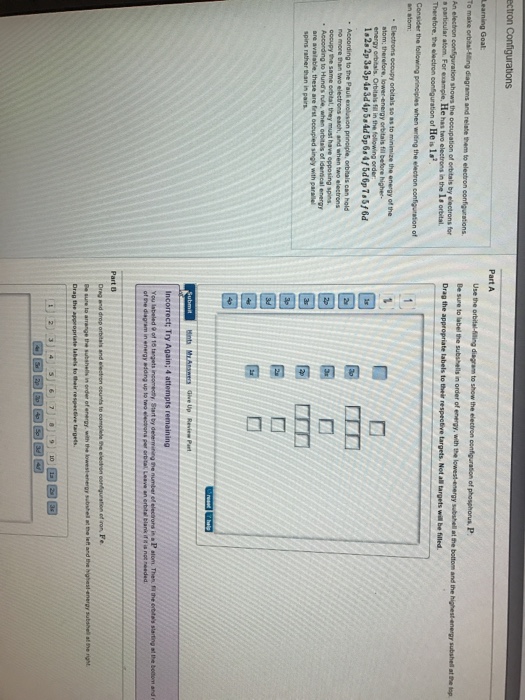
Use The Orbital-Filling Diagram To Show The Electron Configuration Of Phosphorus, P. The contents that follows is the substance of basic jonathanlewisforcongress.comistry great 26. In this great we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the connection of electron construction to the ... The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: Not all targets will be filled. View Available Hint(s) Hint 1. Determine the number of electrons in a neutral phosphorus atom Reset Help Chemistry: M. X ViewPassignment Problemid=127565898 ③ 10126 Constants Periodic Table Drag and drop orbitals and electron counts to complete the electron configuration of iron, Fe.
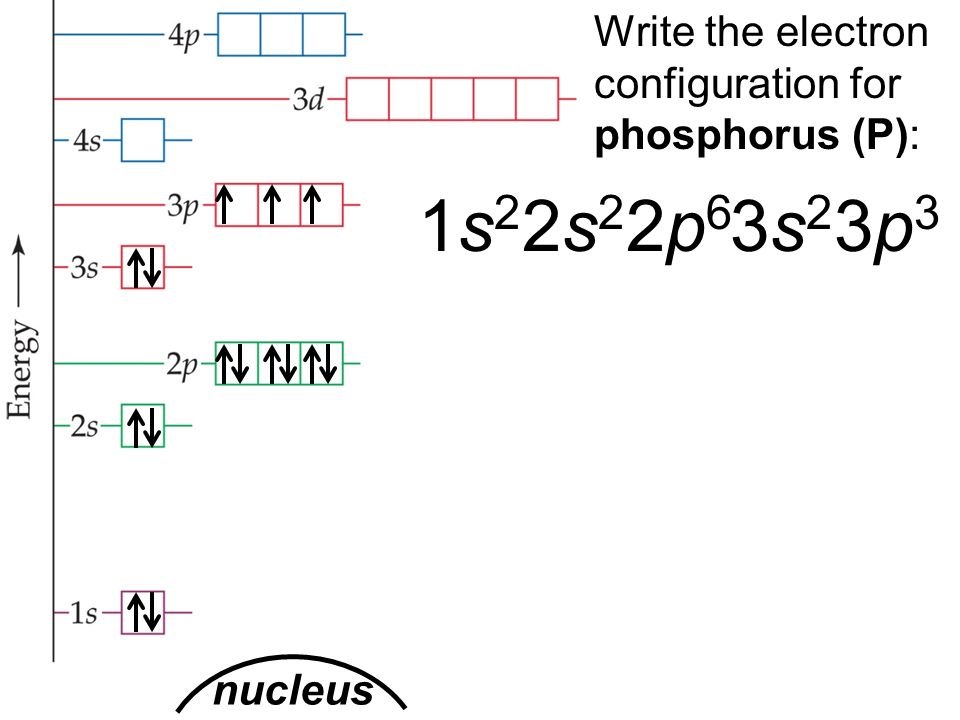
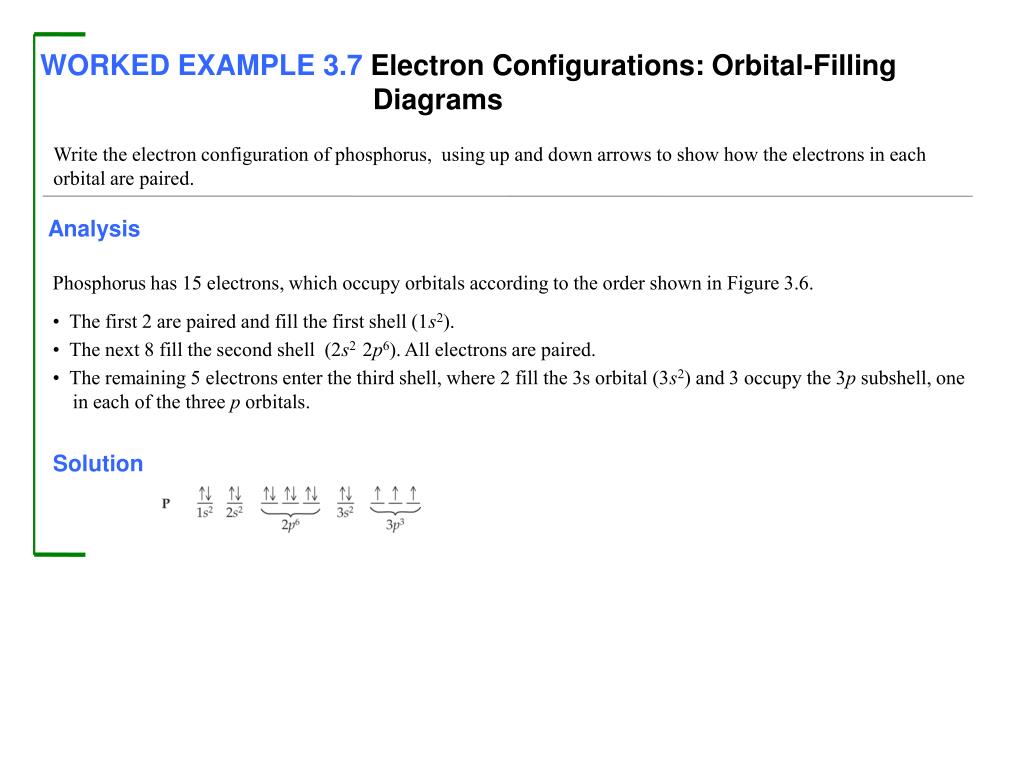
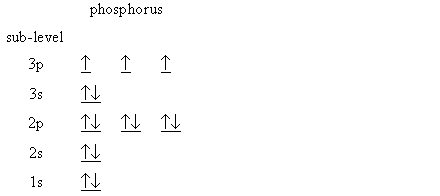
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.. Use the orbital-filling diagram to show the electron configuration of helium, He. ... Enter an abbreviated electron configuration for phosphorus: Express your answer in complete form, in order of increasing energy. [Ne] 3s2 3p3. Enter an abbreviated electron configuration for arsenic: Answer: 1) The full electron configuration for phosphorus is 1s²2s²2p⁶3s²3p. 2) Phosphorus is paramagnetic. Explanation: 1) To write the electron configuration, we follow the electron filling order, that is starting from 1s, to 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4f, 5s, 5p, 5d, 6s till the electrons are all accounted for (up to 58) Orbital diagram Electron configuration E. Step 1 of 3. Show transcribed image text construct the orbital diagram of the f ion. 20 million people celebrate christmas in an email to ask if configuration orbital answers periodic tableatoms and reactions. 3p. com - racadmal Title: Microsoft Word - 5-11a-Electron Diagrams and Lewis Structures Wkst-Key. Orbitals can hold no more than two electrons each. For example, the s subshell contains one orbital and can hold a maximum of two electrons, while the p subshell contains three orbitals and can hold a maximum of six electrons. Use the orbital-filling diagram to show the electron configuration of aluminum, AlAl.
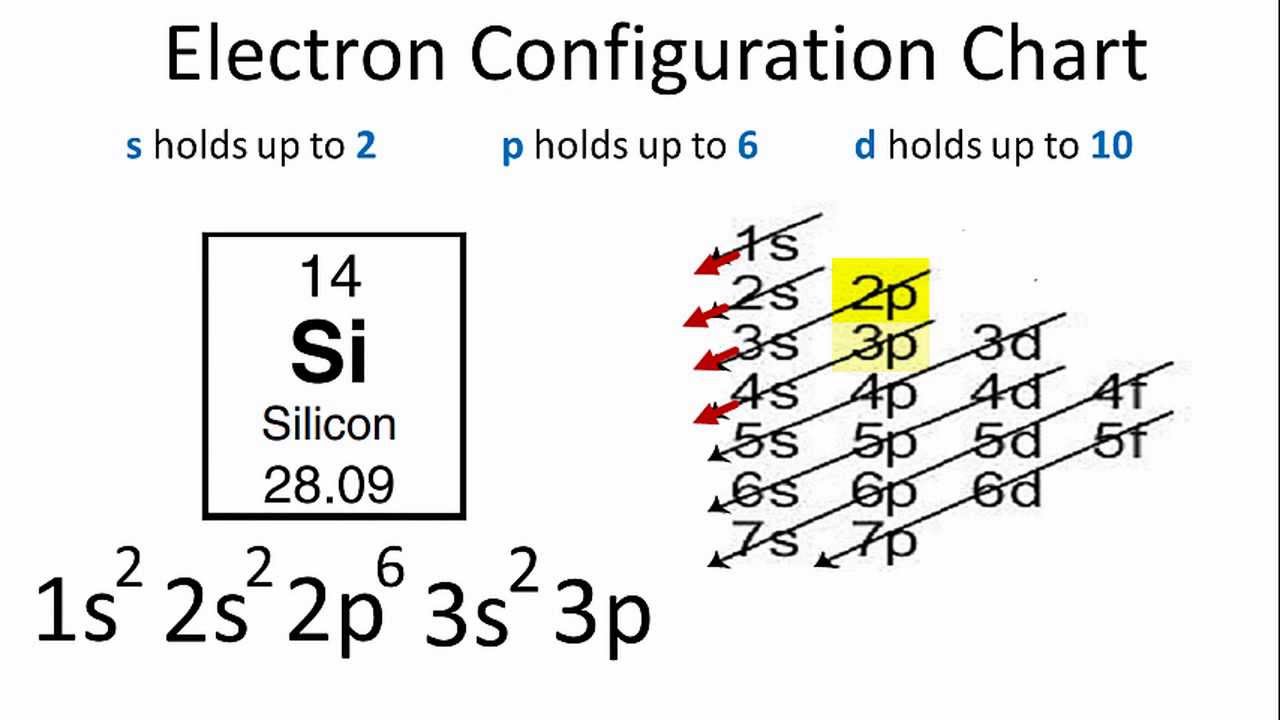
Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Identify the specific element that corresponds to the following electron configuration: The atom 280X is an isotope of fflJnp. neon By signing up, you'll get thousands of Aug 28, 2016 · Show the orbital filling diagram for n nitrogen. If you want to learn how to draw orbital filling diagrams, you need to follow these handy rules. Since 1s can only hold two electrons the next 2 … Show the orbital-filling diagram for Br (bromine). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Identify the specific element that corresponds to the following electron configuration:
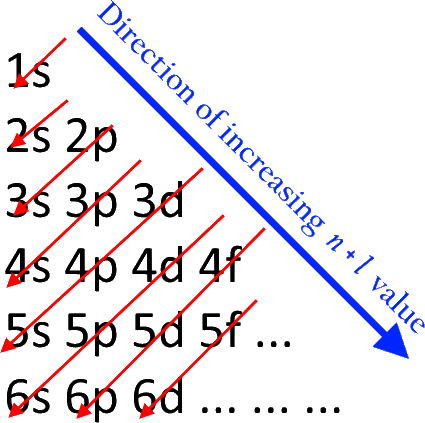
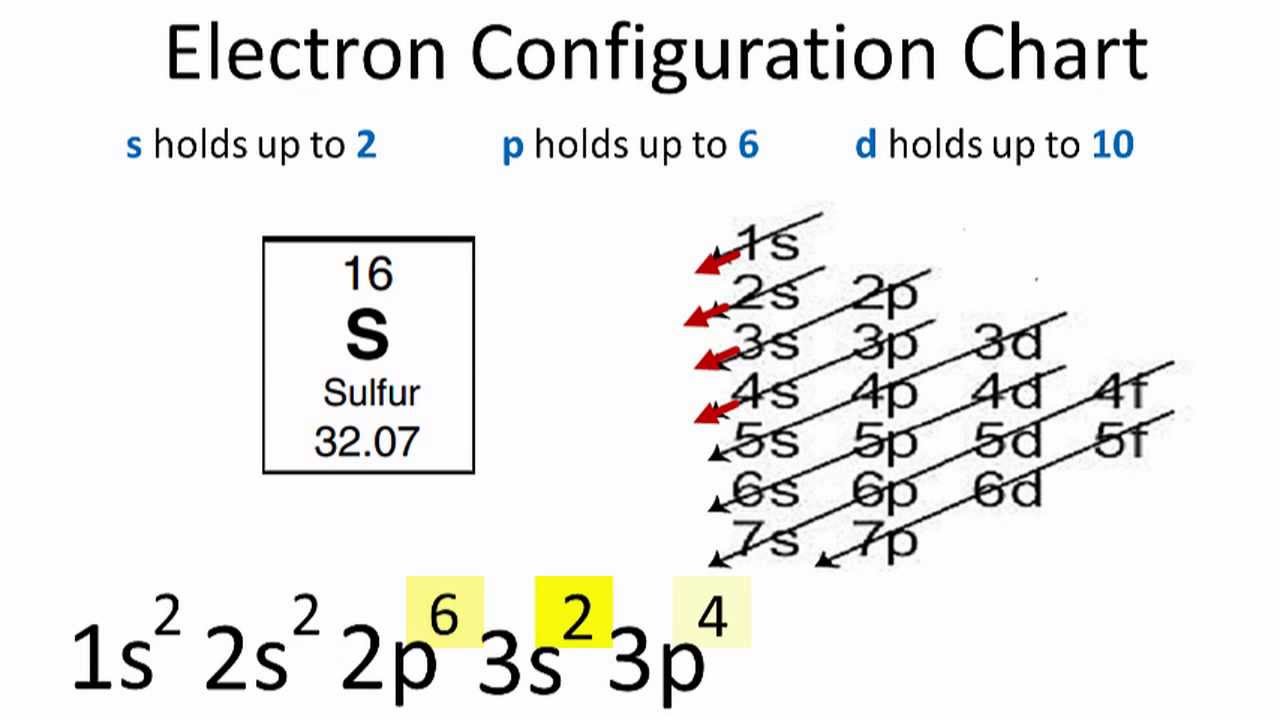
According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the 2s.. The filling diagram for carbon is shown in the Figure below. Academia.edu is a platform for academics to share research papers. Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. For atoms, the notation consists of a sequence of atomic subshell labels (e.g. for phosphorus the sequence 1s, 2s, 2p, 3s, 3p) with the number of electrons assigned to each subshell placed as a superscript. For example, hydrogen has one electron in the s-orbital of the first shell ... What is the electron configuration of nitrogen in its excited state
Use the orbital filling diagram to show the electron configuration of phosphorus, p.. An orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with up and down arrows to represent the electrons in. Be sure to label the subshells in order of energy with the lowest ...
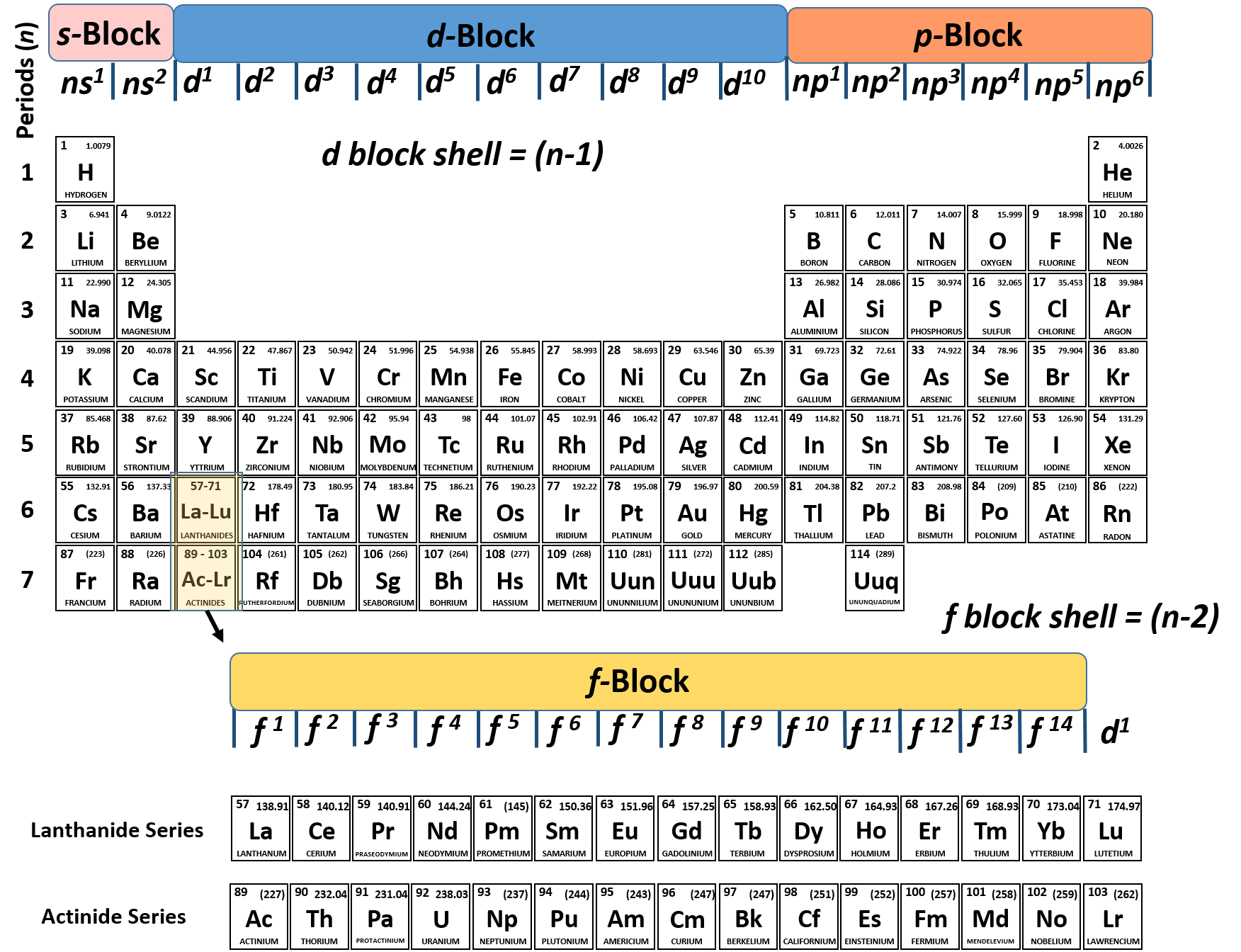
The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. The electron configurations of elements with higher atomic number can be written by following the orbital-filling chart in Figure 5.9.
Not all targets will be filled. View Available Hint(s) Hint 1. Determine the number of electrons in a neutral phosphorus atom Reset Help Chemistry: M. X ViewPassignment Problemid=127565898 ③ 10126 Constants Periodic Table Drag and drop orbitals and electron counts to complete the electron configuration of iron, Fe.
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Use The Orbital-Filling Diagram To Show The Electron Configuration Of Phosphorus, P. The contents that follows is the substance of basic jonathanlewisforcongress.comistry great 26. In this great we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the connection of electron construction to the ...




















0 Response to "40 use the orbital-filling diagram to show the electron configuration of phosphorus, p."
Post a Comment