41 calculate bond order from mo diagram
Use the MO diagram (below) to calculate the bond order for BN. ор 0 ; Question: Use the MO diagram (below) to calculate the bond order for BN. ор 0 . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
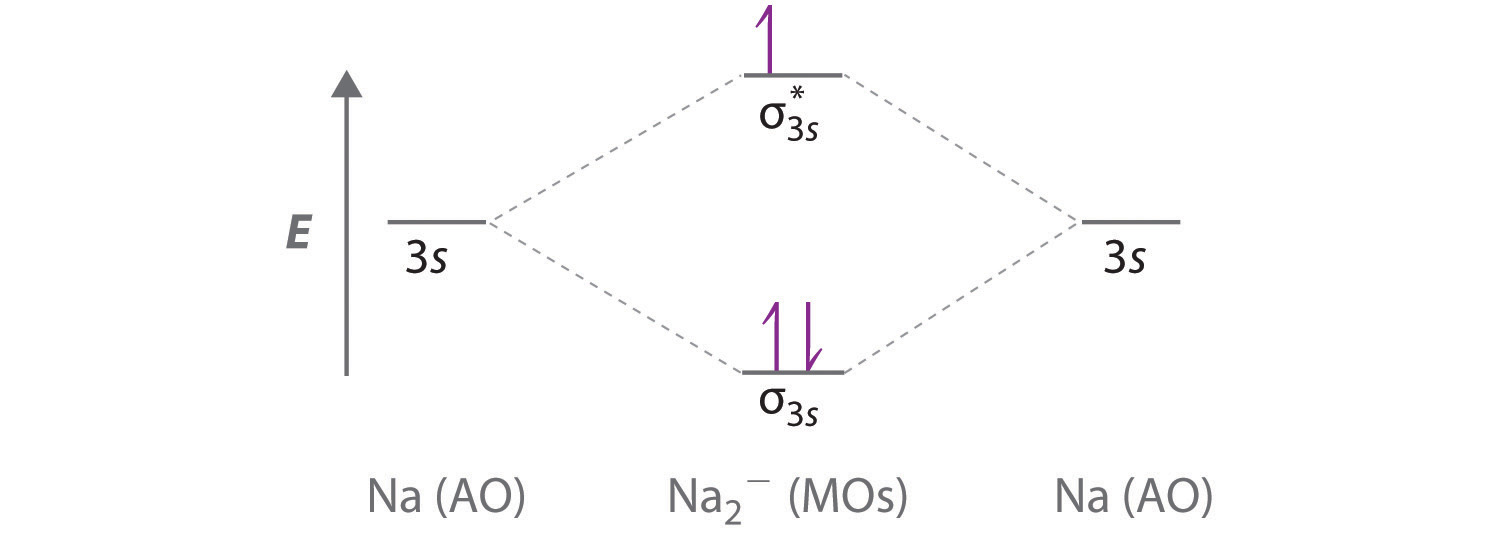
Calculate bond order from mo diagram
Answer (1 of 5): \text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2} Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B.O.=\frac{8-2}{2}=3 Here's a guide on how to construct MO diagrams, i... The bond order describes the stability of the bond. From the completed energy level diagram we can calculate the bond order defined as one half the net number of bonding electrons. 2 know that the higher the bond order the more stable the molecule. Molecular orbital diagram for h2 7. Bond order is the number of chemical bonds between a pair of ... Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ...
Calculate bond order from mo diagram. MO Diagram of I 2-Base Complex ... calculate) the "bond strength" of the I 2-base interaction, in kJ/mol. Compare these values to common covalent bond strengths (for example, a C-C bond, or a Cl-Cl bond) and to the strengths of intermolecular forces (measured as enthalpy of vaporization values). Bond order formula - molecular orbital theory — The molecular orbital theory has never been so clear as with our bond order calculator. In the ... Bond order gives an indication of the stability of a bond. An element with bond order value 0 cannot exist, but compounds can have a 0 bond value. Isoelectronic species have same bond number. Bond order in molecular orbital theory. In molecular orbital theory, bond order is also defined as half the difference between the number of bonding el In the molecular orbital theory, bond length, bond strength, bond order, and magnetic properties of different molecules plays an important role. Bond order is just several electrons that are present between two atoms. One can calculate the bond order of any molecule by creating a molecular orbital diagram like calculate the bond order in h−2.. To create the molecular diagram, you can start ...
A further example is given using the NH3 MO diagram. Here, they calculate the bond order as 3, ignoring the fact that the NH3 a1 orbital is weakly bonding. If we were to in theory calculate the MO diagram for NH3 in a trigonal planar geometry (same as for BH3), we would also get the bond order as 3. A bond order of one is obtained by employing the formula above indicating a stable bond. Bond orders can be calculated from lewis structures which are the heart of the valence bond model. Lecture 10 Part B Mo Diagram Of Nh3 Youtube Bond order no. How to calculate bond order from mo diagram. Molecular orbital mo theory and the bond order. Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O-O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons. Since 1s of H and 2px of F will overlap axially ,then the m.o. formed will be sigma bonding and sigma*. Both these electrons will be filled in sigma s-px molecular orbital. Rest atomic orbitals of F will not form any m.o.and will maintain their atomic character. HF = K , (2s2) , (2py2) , (2pz2) , sigma 2px 2 ,sigma spx 2 , sigma* spx0.
What you need to understand are the periodic trends and how to affect bond lengths. When calculating bond orders, you use the equation Bond order= [(# of bonding electrons)-(# of antibonding electrons)]/2. The way you find the number of bonding and antibonding electrons is by understanding and using the appropriate molecular orbital diagram. The bond order in sulfur dioxide, for example, is 1.5 the average of an S-O single bond in one Lewis structure and an S=O double bond in the other. In molecular orbital theory, we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital ... write the molecular orbital diagram of n2 and calculate their bond order - Chemistry - TopperLearning.com | qbqjy Practice Test - MCQs test series for Term 1 Exams ENROLL NOW In this example problem, we show how to fill a molecular orbital diagram for a diatomic molecule and use molecular bond theory to compare bond order, bond st...
Click here 👆 to get an answer to your question ️ How to calculate bond order from molecular orbital diagram? halexisjennah halexisjennah 02/13/2017 Chemistry High School answered How to calculate bond order from molecular orbital diagram? 1 See answer Advertisement
📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at http://conquerchemistry.com/chem-secrets/💯 If you like my teaching style and are inte...
As bond order in Oxygen is 2 so two bonds i.e. double bond is formed between two oxygen atoms (O=O). Further more as there are two unpaired electrons in Oxygen molecule hence it is paramagnetic. Also Watch Molecular orbital diagram of O2 , O2 +2, 02 - 2 ( in Urdu / Hindi) Simplest Trick to Calculate Bond Order :
Describe traits of bonding and antibonding molecular orbitals; Calculate bond orders based on molecular electron configurations; Write molecular electron ...
Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. If you calculate their bond order, you get: BOH+ 2 = 1 2(Bonding − ...
Bond Order in Molecular Orbital Theory — The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (1-0)/2 ...Molecular Orbitals Involving... · Energy-Level Diagrams
A molecular orbital diagram is an important tool used for explaining the bonding present in the molecules. With the help of a molecular orbital diagram, we can easily determine the bond order and ...
The bond order of the formed bond can be calculated from the MO diagram as follows: bond order = (number of bonding electrons)- (number of anti-bonding electrons) 2 bond order = (number of bonding ...
To determine the bond order of a diatomic molecule such as H 2, CO or HCl, you simply look at the kind of bond involved and that is your answer. A molecule of hydrogen gas (H 2) has single bond and a bond order of 1. A molecule of oxygen gas (O 2) has a double bond and a bond order of 2. The triple bond of CN gives it a bond order of 3.
4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3.
The bond order shows the number of chemical bonds present between a pair of atoms. For instance, the bond order of diatomic nitrogen N≡N is 3 and bond order between the carbon atoms in H-H≡C-H is also three. The bond order describes the stability of the bond. The molecular orbital provides an easy understanding of the concept of the bond ...
The bond order of N2 (nitrogen) - nitrogen molecule has a triple bond, so the bond order is three. Step 1. Write the electron configuration of a N2 molecule. Electronic configuration of N2 = (σ2s)2 (σ*2s)2 n (2px)2 n (2py)2 n (2pz)2. Step 2. From the above electron configuration, put the values in the formula.
Bond order is also an index of bond strength, and it is used extensively in valence bond theory. Dihydrogen (H 2) This MO diagram depicts the molecule H 2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely occupied. A bond order of one is obtained by employing the formula above ...
5 Dec 2017 · 8 steps1.Know the formula. In molecular orbital theory, bond order is defined as half of the difference between the number of bonding and antibonding electrons. Bond ...2.Know that the higher the bond order, the more stable the molecule. Each electron that entered a bonding molecular orbital will help stabilize the new ...3.Consider a simple example. Hydrogen atoms have one electron in the s shell, and the s shell is capable of holding two electrons. When two hydrogen atoms ...
Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ...
The bond order describes the stability of the bond. From the completed energy level diagram we can calculate the bond order defined as one half the net number of bonding electrons. 2 know that the higher the bond order the more stable the molecule. Molecular orbital diagram for h2 7. Bond order is the number of chemical bonds between a pair of ...
Answer (1 of 5): \text{Bond order} = \frac{n_{\text{bonding electrons}}-n_{\text{antibonding electrons}}}{2} Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B.O.=\frac{8-2}{2}=3 Here's a guide on how to construct MO diagrams, i...



0 Response to "41 calculate bond order from mo diagram"
Post a Comment