41 fractional distillation phase diagram
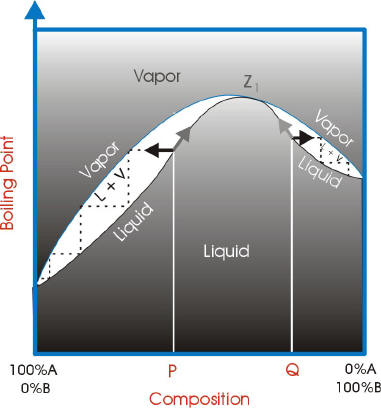
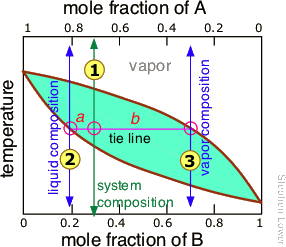
The figure shows the phase diagram of a system in which the liquids become fully miscible before they boil. Distillation of a mixture at a 1 leads to vapor with composition b 1, which condenses to completely miscible solution at b 2. Phase separation only occurs when the distillate is cooled to a point in the two-phase region such as point b 3. This diagram shows the purification power vs. the number of trays in a practical environment. Pressure Drop. This is primarily relevant in vacuum fractionation or fractional distillation. In this context, 'pressure drop' refers to the difference in pressure between the boiling flask and the head of the column.
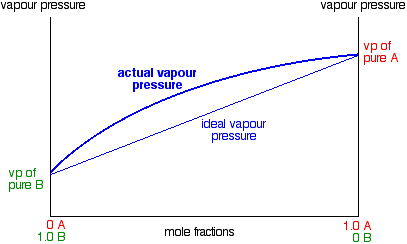
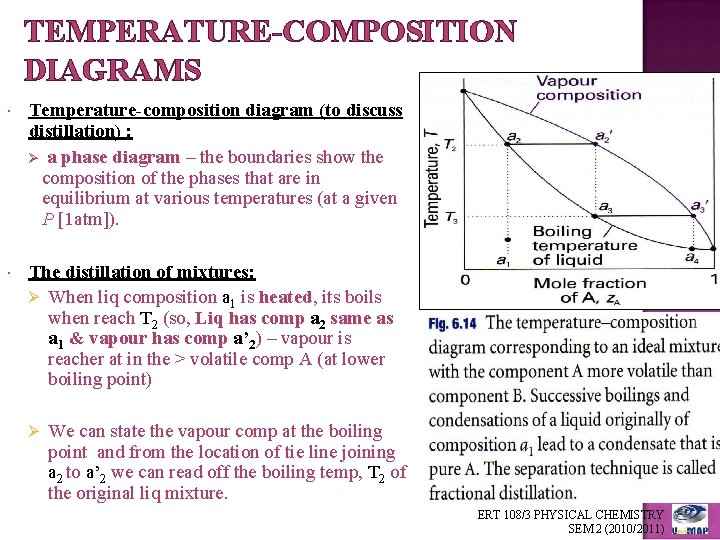
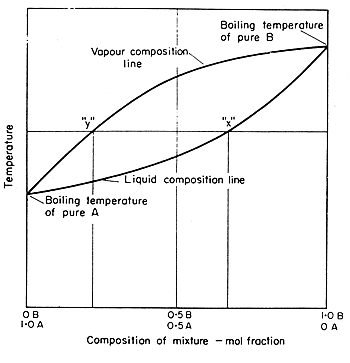
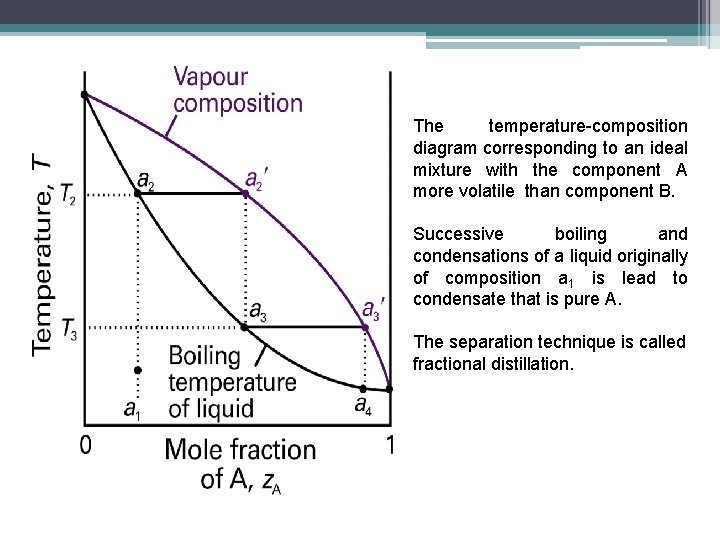
temperature. These diagrams are needed when the aim is to separate the two liquids by fractional distillation. Figure 1 shows the phase diagram for an ideal solution. In a distillation experiment with constant pressure, the solution is heated and steam is extracted and condensed. The condensed liquid is richer in the more volatile
Fractional distillation phase diagram
This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Using the phase diagram On the last page, we looked at how the phase diagram for an ideal mixture of two liquids was built up. Fractional distillation utilizes a packed column before the still head to give an increased ... bench one piece at a time using plastic clamps as shown in the diagram (you will use three plastic clamps). ... phase and a gaseous mobile phase called the carrier gas. While the sample molecules are in the Fractional distillation is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.
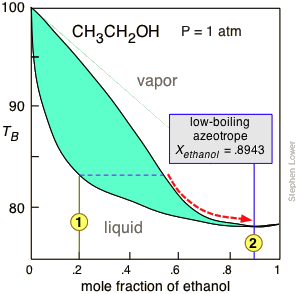
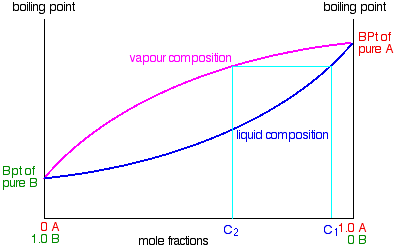
Fractional distillation phase diagram. The previous relationships of alcohol-water mixtures hold true up to alcohol concentrations of about 95.6 percent. At this concentration, the two substances quit boiling separately (i.e., the alcohol in the vapor phase is no longer more concentrated than in the liquid phase), and fractional distillation no longer works. A phase diagram can be used to discuss the process of fractional distillation 2. Depending on the relative strengths of the intermolecular forces, high- or low-boiling azeotropes may be formed 3. The vapor pressure of a system composed of immiscible liquids is the sum of the vapor ... As the distillation proceeds, the mixture in the pot becomes more and more rich in 1-butanol. If we made a boiling point-composition diagram (or phase diagram) for a 50:50 mixture of acetone and 1-butanol (see Fig. 4.2), we would see from the diagram, looking at the upper Description
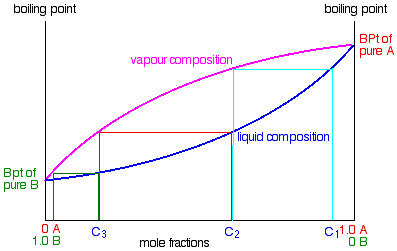
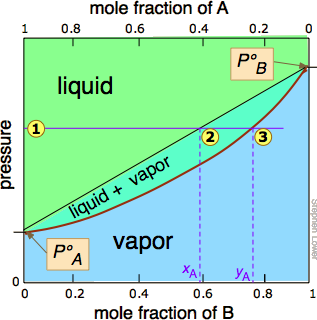
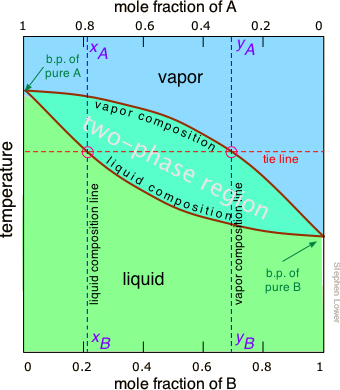
the vapor phase. C) A fractional distillation is, in effect, multiple distillations in the same setup. The first distillation is in the distillation flask. The other distillations are in the fractionating column. D) The GC detector responds differently to each compound. Thus, the areas of the peaks are not directly Pressure-swing distillation takes advantage of the fact that boiling point (T,X) diagrams are two-dimensional slices of a (T,X,P) diagram in which the pressure is the third variable. This means that the azeotropic composition depends on the pressure, so distillation at some pressure other than 1 atm may allow one to "jump" the azeotrope. P-xy T-xy Diagrams. Definition: The P-xy and the T-xy are diagrams that represent the liquid and vapour equilibrium for a binary mixture. The component that is graphed is the most volatile one because is the one that will evaporate first during the distillation process. On the x-axis goes the mole fraction x,y (for liquid phase and vapour phase ... To understand the nature of simple distillation, fractional distillation and azeotropes we need to look at vapor/liquid diagrams for pairs of solvents. The graph below (Fig. 5) shows such a diagram for 2 solvents, A and B. A is the lower boiling material. The bottom of the graph shows the liquid state and the top of the graph shows the vapor state.
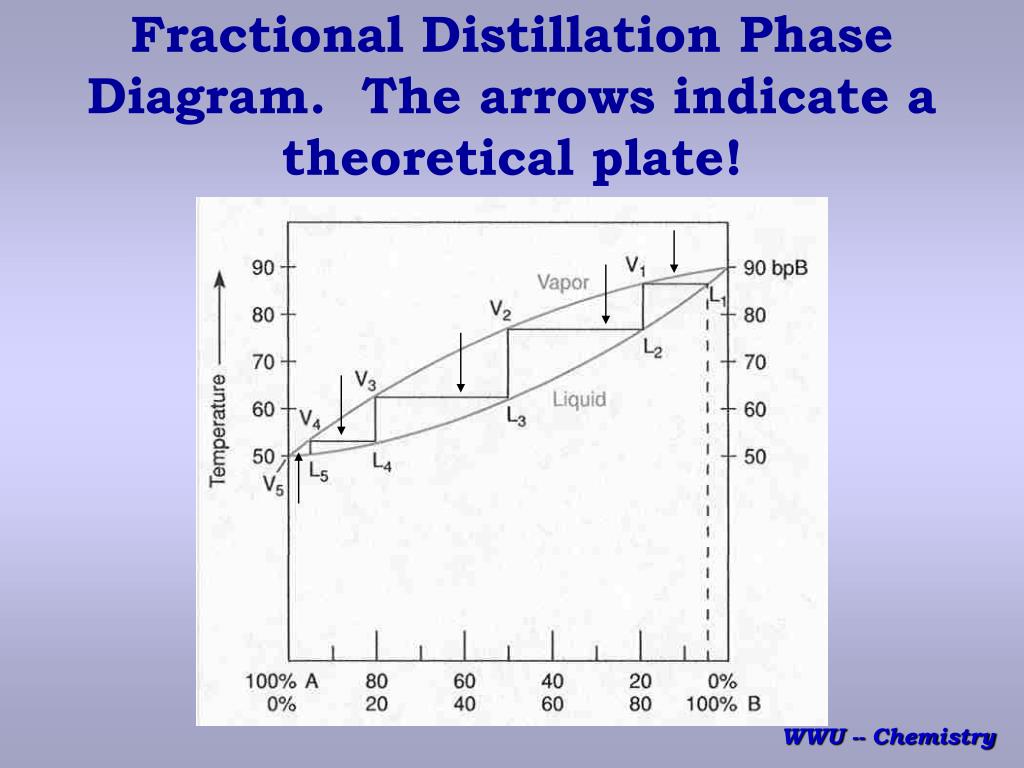
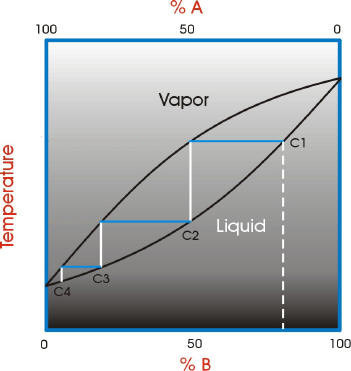
Looks at how this affects the fractional distillation of such mixtures. NON-IDEAL MIXTURES OF LIQUIDS This page looks at the phase diagrams for non-ideal mixtures of liquids, and introduces the idea of an azeotropic mixture (also known as an azeotrope or constant boiling mixture). CHM220 Distillation Lab Page 4 of 7 Figure 5b Fractional Distillation Phase Diagram. The arrows indicate a theoretical plate. Each vaporization is represented by a horizontal line connecting the liquid composition curve to the vapor composition curve. Each condensation is represented by a vertical line connecting the Distillation Distillation is a commonly used method for purifying liquids and separating mixtures of liquids into their individual components. Familiar examples include the distillation of crude fermentation broths into alcoholic spirits such as gin and vodka, and the fractionation of crude oil into useful products such as gasoline and heating oil. Phase diagrams for fractional distillation. I learned about phase diagrams involving partial Vapour composition, temperature and composition of binary solutions from this website. You can find it if you scroll down to a little above the end. First it considers a binary solution of volatile liquids. The diagram involves boiling point on Y-axis ...
Fractional Distillation: Fractional distillation is a very commonly used technique for separating mixtures of liquids. It can be quite complicated in application, especially if azeotropes are present. ... A better way of doing this is to use a phase diagram such as the one now shown.
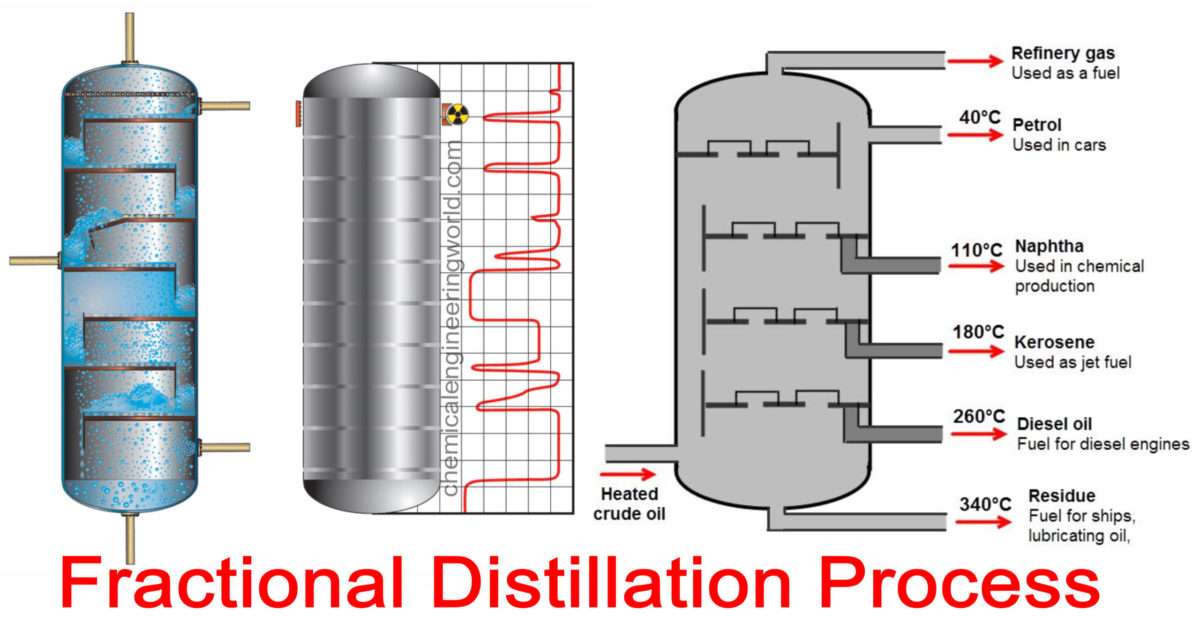
Applications of Fractional Distillation. Fractional distillation is useful for the production of high-purity silicon from chlorosilanes. Silicon is very much in use in semiconductors. It is in use for the separation of liquefied air. We obtain components like liquid nitrogen, concentrate argon and oxygen.
Fractional crystallization is a stagewise separation technique ... attainable by distillation. The phase diagrams clearly demon-strate that pure A and pure B crystals can in principle be obtained over a range of temperatures and concentrations as indicated by the corresponding free-
Fractional distillation is a process by which individual components can be separated using heat from a given mixture. The boiling points of each component in the mixture determine the order of ...
Fractional distillation industrially. There is no difference whatsoever in the theory involved. All that is different is what the fractionating column looks like. The diagram shows a simplified cross-section through a small part of a typical column. The column contains a number of trays that the liquid collects on as the vapour condenses.
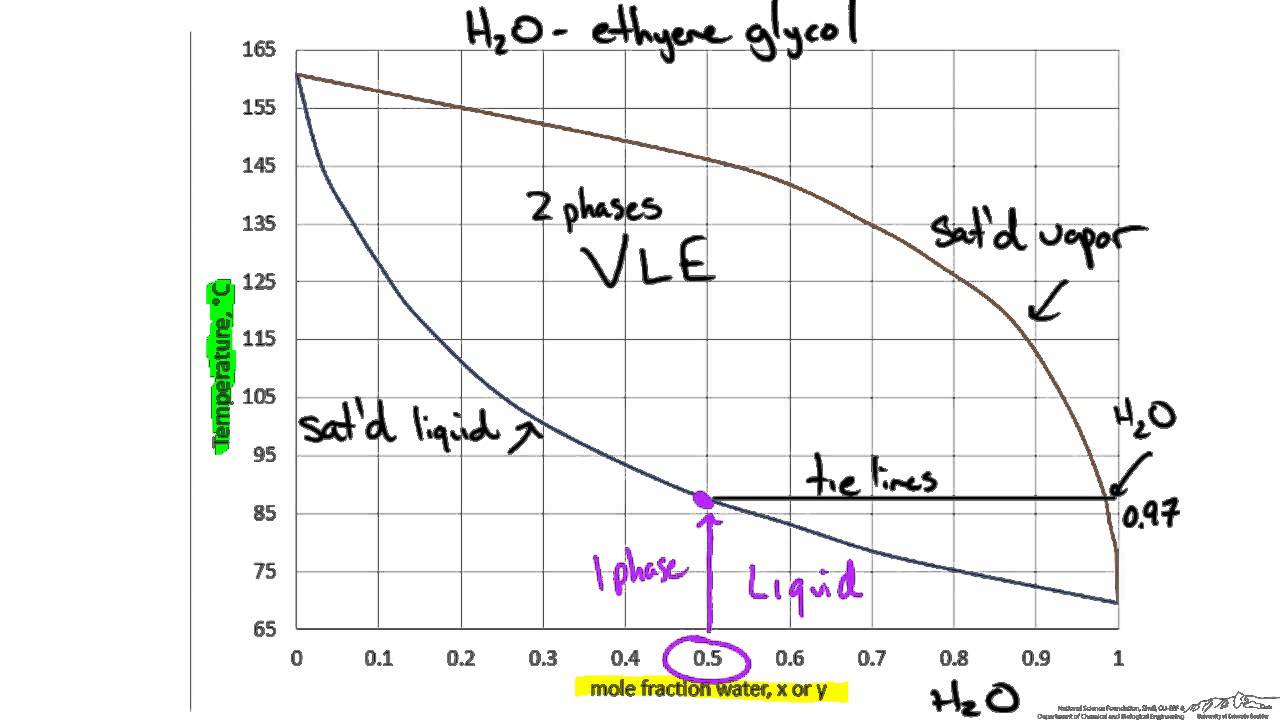
phase diagrams of mixtures Gibbs phase rule liquid-vapor distillation azeotropes Review osmotic pressure balance ∆G from mixing and from pressure increase −= RT X ∫+ V dp Amp p ln Π ΠV = nBRT Debye-Hückel Theory We assume: 1) the solvent is a perfect continuum dielectric (ε ~ 80 for water, ε = 1 for vacuum)
This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. This is the second page in a sequence of three pages. Important: If you have come straight to this page from a search engine and are looking for simple ...
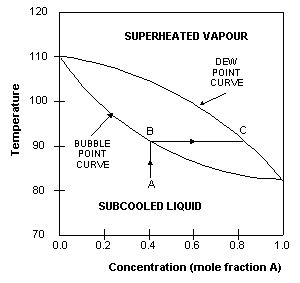
Distillation is method of separation of components from a liquid mixture which ... phase diagram is more commonly used in the analysis of vapor-liquid equilibrium. Figure 5.1: Phase diagram of binary system at constant pressure 5.1.4. Constant temperature (isothermal) phase diagram
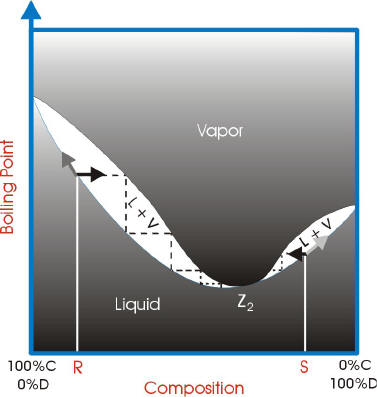
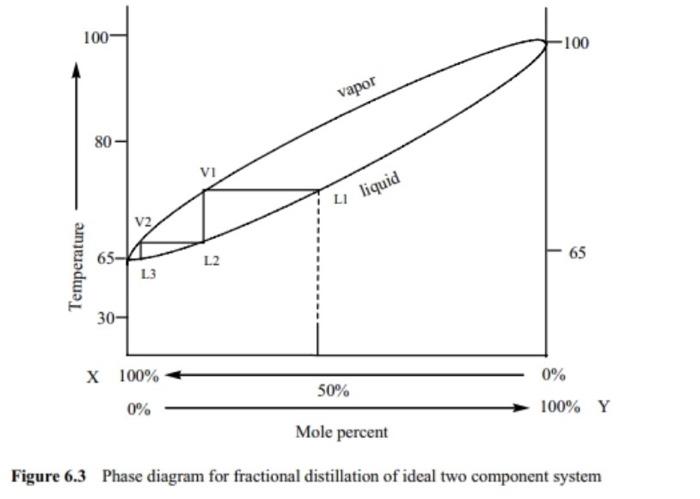
A phase diagram is a plot of composition (mol %) vs. temperature (T), where the lower ... Figure 5: Fractional Distillation of a simple two component mixture of initial composition 5%A, 95%B where A is the lower boiling liquid, and B is the higher boiling liquid. 25°C. Due to this continuous enrichment of the vapor, the temperature behavior of ...
Most asked question in interview./RefineryUnderstand fractional distillation with graphLink : fractional DistillationA short overview of how fractional dist...
Simple and fractional distillations. Transcript. Learn how chemicals can be separated and purified through distillation, a process which separates them based on their respective boiling points. By Angela Guerrero. . Created by Angela Guerrero. Methods of purification of organic compounds.
Fractional distillation is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.
Fractional distillation utilizes a packed column before the still head to give an increased ... bench one piece at a time using plastic clamps as shown in the diagram (you will use three plastic clamps). ... phase and a gaseous mobile phase called the carrier gas. While the sample molecules are in the
This page explains how the fractional distillation (both in the lab and industrially) of an ideal mixture of liquids relates to their phase diagram. Using the phase diagram On the last page, we looked at how the phase diagram for an ideal mixture of two liquids was built up.

Fractional Distillation Distilled Water Petroleum Seawater Angle Chemistry Drinking Water Png Pngwing
An Azeotropic Mixture Of Water B Pt 100 C And Hcl Bp T 85 C Boils At 108 5 C When This Mixture Is Distilled Is It Still Possible Quora













0 Response to "41 fractional distillation phase diagram"
Post a Comment