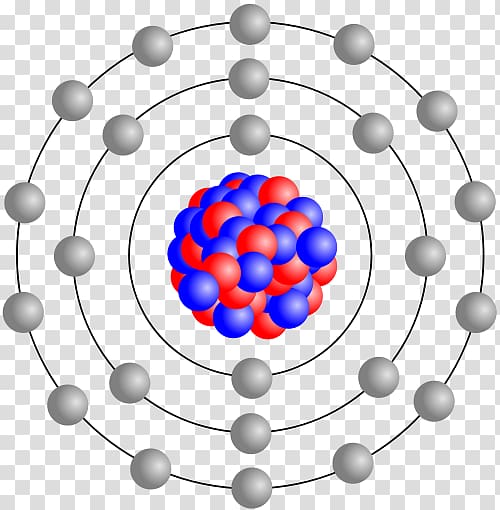
42 bohr diagram for phosphorus
Bohr won a Nobel Prize in Physics for his contributions to our understanding of the structure of atoms and how that is related to line spectra emissions. Key Concepts and Summary. Bohr incorporated Planck's and Einstein's quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons.
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.

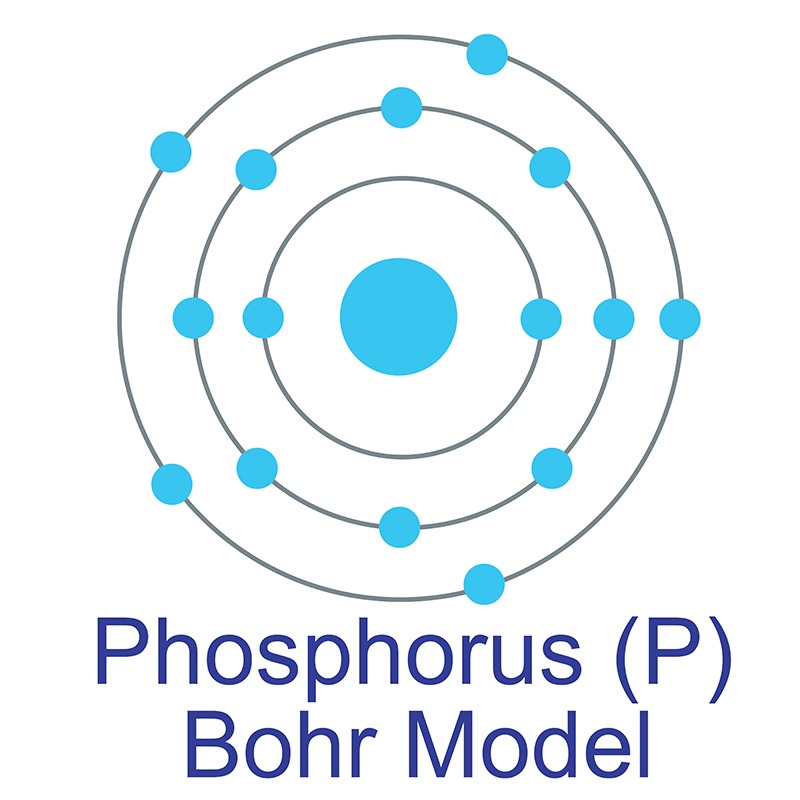
Bohr diagram for phosphorus
24.09.2019 · In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen . 1 proton. 1 electron. 0 neutrons . You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom. In the model for the hydrogen atom, a negatively … 05.11.2019 · It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For that, we have electron shell diagrams.. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.. For each electron shell atom diagram, the element symbol is listed in the nucleus. The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. == The phosphorus bohr model has 3 ...
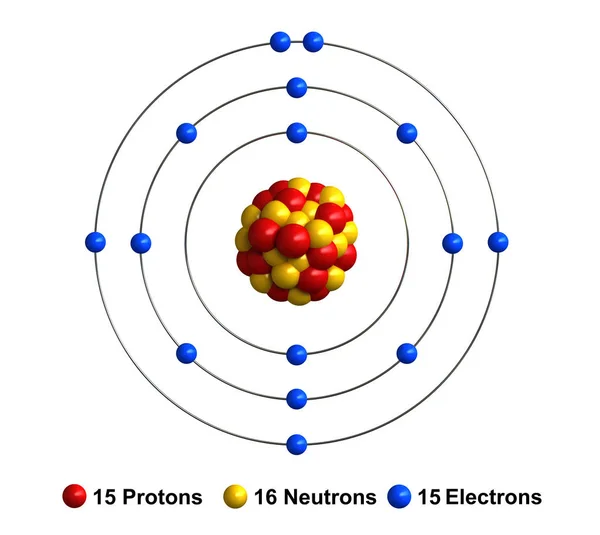
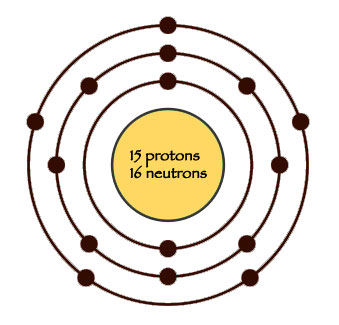
Bohr diagram for phosphorus. Bohr-Rutherford Diagrams of Ions ... Try to make a Bohr-Rutherford ion for phosphorous. 31 P 15 3-Metals will often form bonds with non metals. This is the Basis for Ionic compounds The Bohr Model of Phosphorus(P) has a nucleus that contains 16 neutrons and 15 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Phosphorus contains 5 electrons that also called valence electrons. Apr 24, 2017 · To calculate the electron configuration for phosphorus (P), which is in the third row, p-block, third element in that block, write: 1s2 2s2 2p6 3s2 3p3. Check your work by adding the electron numbers to see if they equal the atomic number of the element; for this example, you would write: 2+2+6+2+3=15, which is the atomic number of phosphorus. Find step-by-step Biology solutions and your answer to the following textbook question: (a) Draw the Bohr-Rutherford diagram (without neutrons) for an atom of each of the following elements: lithium, oxygen, calcium; and phosphorus. (b) Draw the; Bohr-Rutherford diagram (without neutrons) for the ion formed by each of the elements in (a). (c) Write the chemical symbol for each ion.
Nitrogen has 2 electrons in its first shell and 5 in its second.Check me out: http://www.chemistnate.com Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. What is the Bohr Model diagram for Phosphorus? on the first shell put two electrons (one on top and one on the bottom)on the lines for the shell. on the second shell put 8 electrons on the lines ... Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
Drawing Dohr model diagrams 1. Refer to the Bohr model chart on page 32 to help you complete the following table. Some answers are provided for you. (Hint: Remember that the maximum number of electrons in the first three shells is 2, 8, and 8.) Number of electrons 10 10 10 14/ 18 18 Number of electron shells Bohr Model of Silicon Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. There are That box on the left has all of the information you need to know about one element.Phosphorus orbital diagram together with atomic structure bohr modelspdf bohr model worksheet duplin county schools various fillable forms further 5 furthermore cgvyb3hpzgugaw9u as well as as well as six types of energy also lewis dot diagram for ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
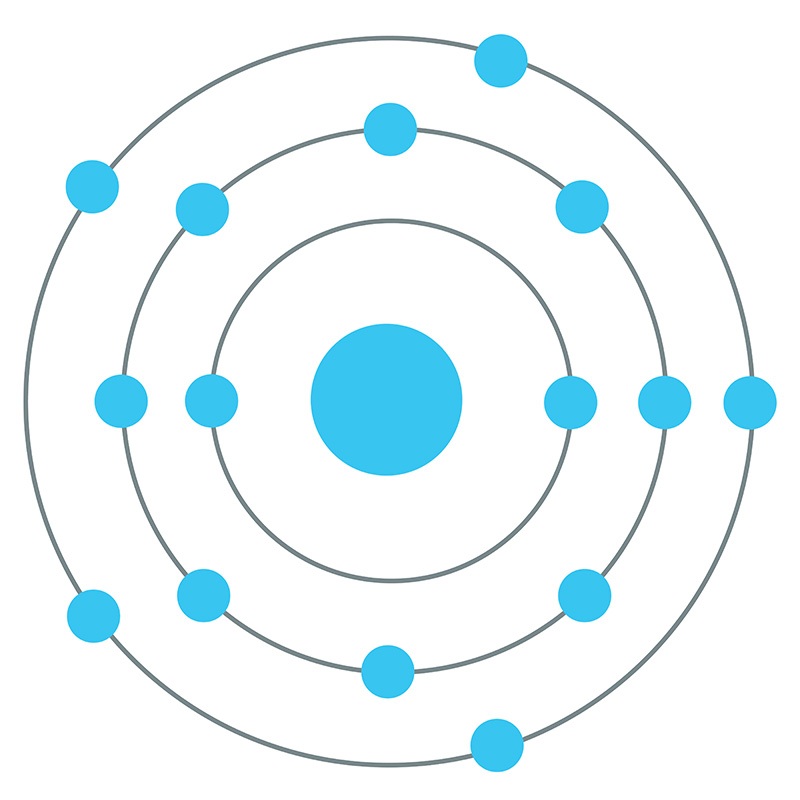
07.03.2021 · Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of Aluminum (Al) 2, 8, 3: 14: Bohr model of Silicon (Si) 2, 8, 4: 15: Bohr model of Phosphorus (P) 2, 8, 5: 16 ...
bohr diagram for phosphorus. For unlimited access to Homework Help, a Homework+ subscription is required.
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, boiling points ...
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box ... You should already be familiar with the Bohr Model of the Atom which states that electrons exist in discrete energy levels (or electron shells). The principal quantum number, n, tells us the energy level (or electron shell) that the electron is found in. n=1 is the first energy ...
The electrons on a Bohr Model have a limit of 2 electrons in the first shell, 8 electrons in the second shell, 8 electrons in the third shell, and so on. The electrons have to be placed specifically like a clock. The first electron is placed on 12:00, the second on 6:00, the third on 3:00, the fourth on 9:00, and back to 12:00.
1024x768 phosphorus bohr diagram awesome bohr diagram phosphorus phosphorus - Bohr Model Drawing Oxygen. 0 0. 335x270 what is a bohr rutherford diagram cute bohr model of magnesium - Bohr Model Drawing Oxygen. 0 0. 261x281 luxury pics of fluorine bohr diagram diagram with labels - Bohr Model Drawing Of Oxygen. 0 0.
We collected 40+ Bohr Model Drawing Oxygen paintings in our online museum of paintings - PaintingValley.com. ADVERTISEMENT. LIMITED OFFER: Get 10 free Shutterstock images - PICK10FREE. oxygen. model. bohr. diagram. atomic. rutherford.
Bohr diagram for phosphorus. 2 electrons can go in the first shell 8 in the second 8 in the third and so on. How to make a 3d model of phosphorus atom . Feb 23 2015 a basic animation of the phosphorus atom including element properties it s date of discovery and location on the periodic table.
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is ‘Na’. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Phosphorus: Phosphorus has 15 protons and is located in group 5A of the periodic table. It is a non-metal and highly reactive. As a result, in nature it is usually found reacted with metals as ...
01.09.2021 · Bohr Diagram For Phosphorus. Electron Configuration Notation: shows the arrangment of electrons around the nucleus of an atom. – helps chemist understanding how elements form chemical. Neon Atom Model, 5th Grade Science Projects, 8th copper bohr diagram wedocable – 28 images – copper element protons and. As was mentioned before, a neutral Phosphorus Atom contains five …
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
The Bohr Model of Sulfur(S) has a nucleus that contains 16 neutrons and 16 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sulfur contains 6 electrons that also called valence electrons.
Bohr Diagram for Phosphorus. what is the bohr model diagram for phosphorus answers == the phosphorus bohr model has 3 shells because it has 15protons and 16 neutrons 30 97 is about 31 so 31 15protons = 16 neutrons the number of how to draw bohr rutherford diagrams phosphorous how to draw the bohr rutherford diagram for phosphorous 2 electrons can go in the first shell 8 in the second 8 in the ...
How to draw the Bohr-Rutherford Diagram for Phosphorous. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
Bohr Diagram Lewis Structure. Title: Thanks! Author: Tracy Trimpe Created Date: 10/30/2002 10:35:02 PM ...
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. == The phosphorus bohr model has 3 ...
05.11.2019 · It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For that, we have electron shell diagrams.. Here are electron shell atom diagrams for the elements, ordered by increasing atomic number.. For each electron shell atom diagram, the element symbol is listed in the nucleus.
24.09.2019 · In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen . 1 proton. 1 electron. 0 neutrons . You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom. In the model for the hydrogen atom, a negatively …











:max_bytes(150000):strip_icc()/phosphorusatom-58b6025c5f9b5860464c65dc.jpg)

0 Response to "42 bohr diagram for phosphorus"
Post a Comment