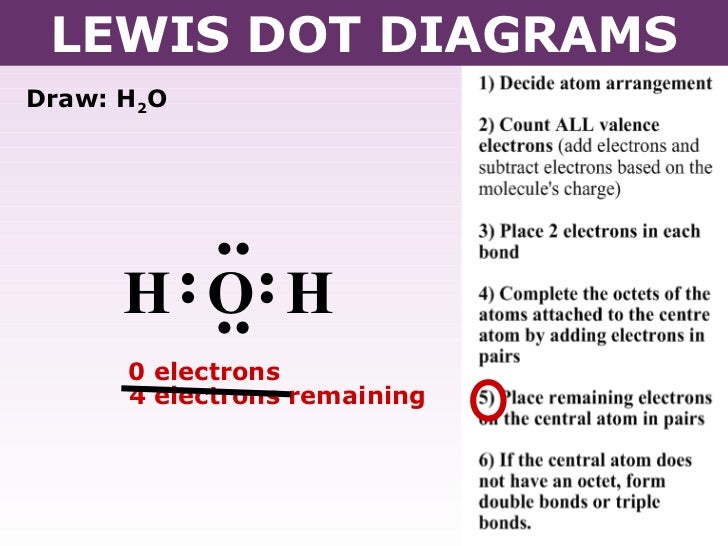
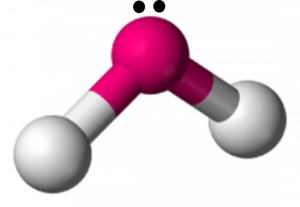
37 dot diagram for h2o
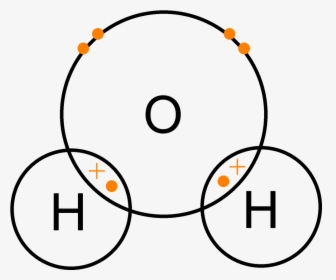
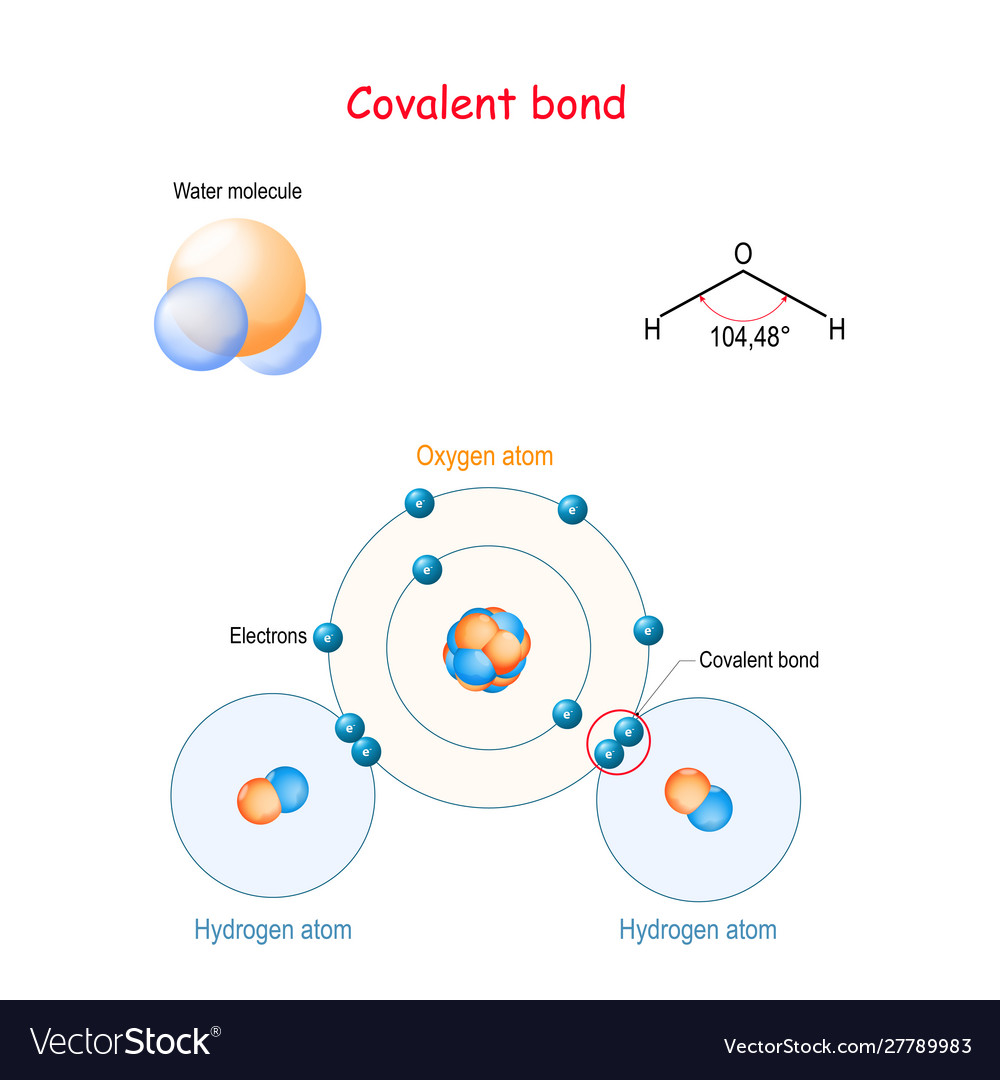
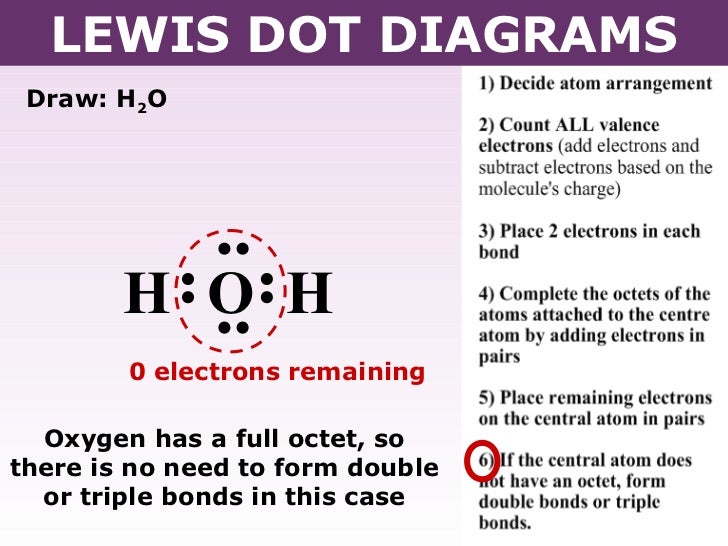
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. H 2 O. Back. 70 More Lewis Dot Structures. Since all the atoms are in either period 1 or 2, this molecule will adhere to the octet rule. The exception, of course, being the hydrogen's. They follow the duet rule (2 electrons). Water is a transparent, tasteless, odorless liquid at room temperature and standard pressure. Water is a polar molecules.
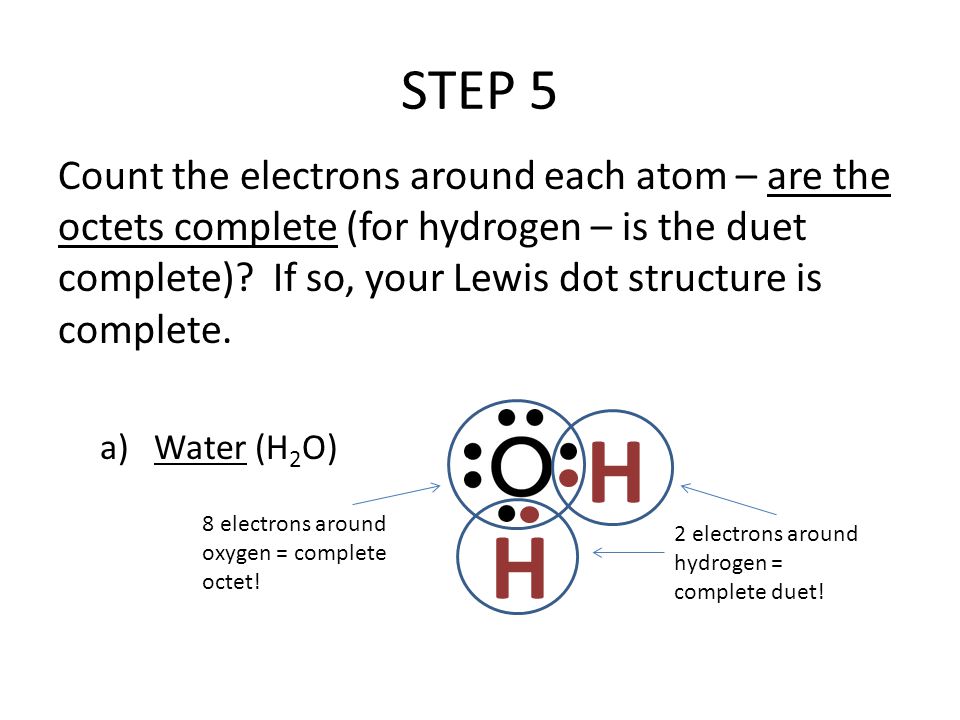
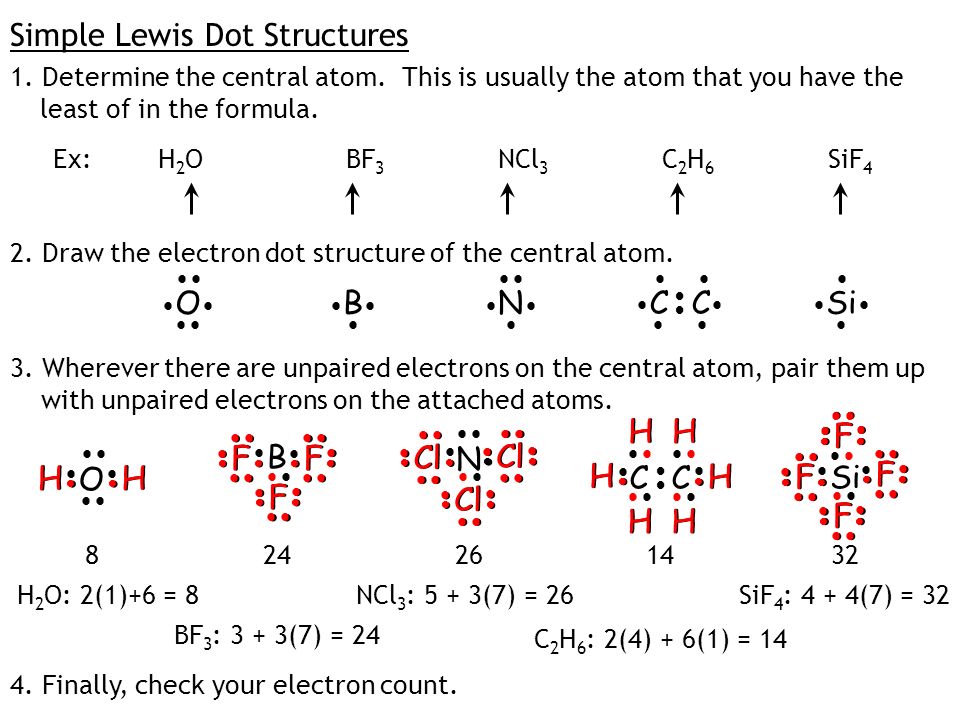
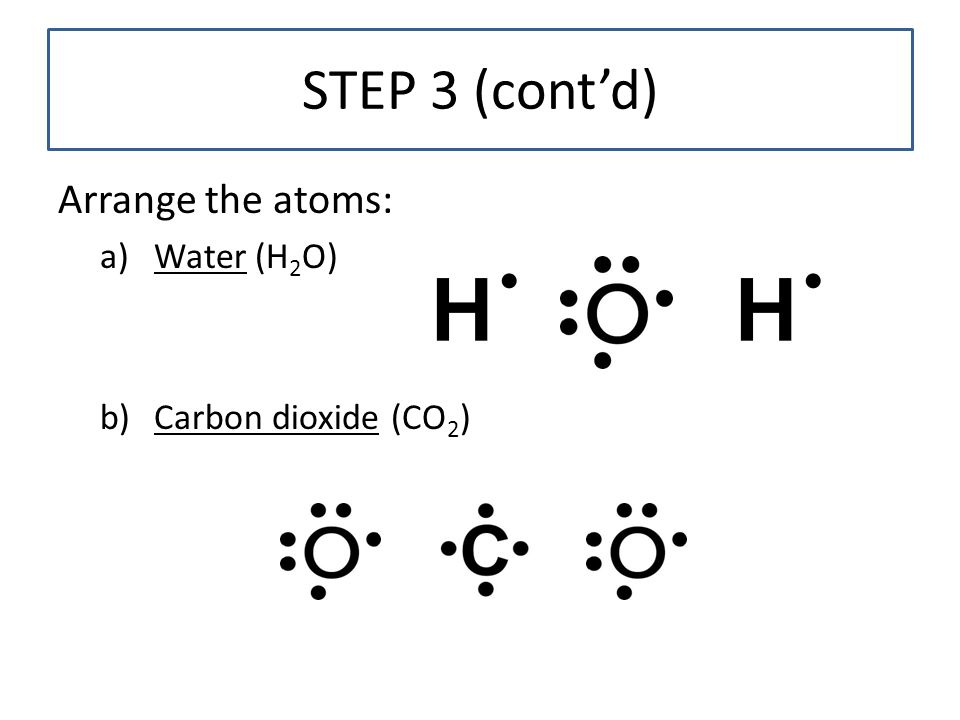
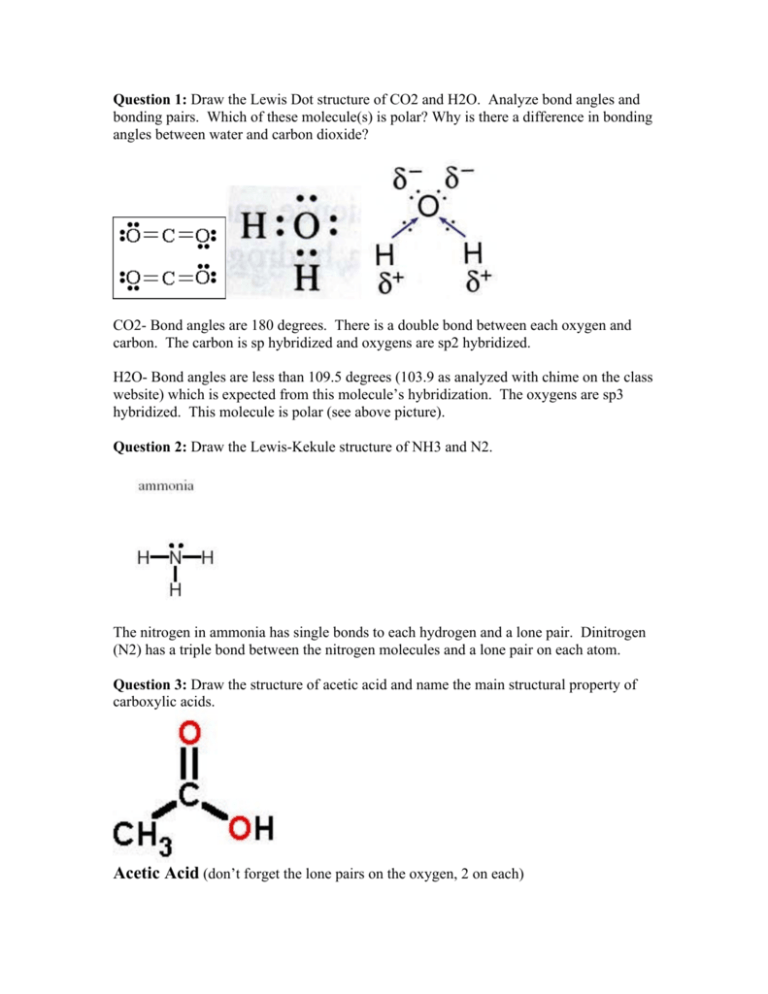
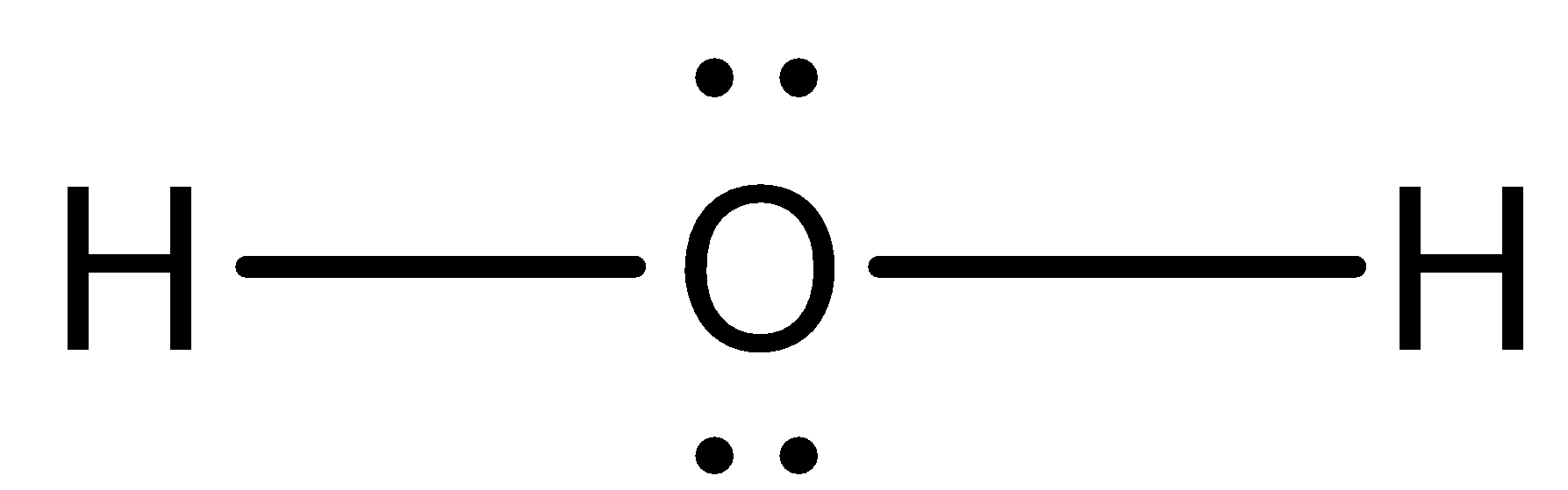
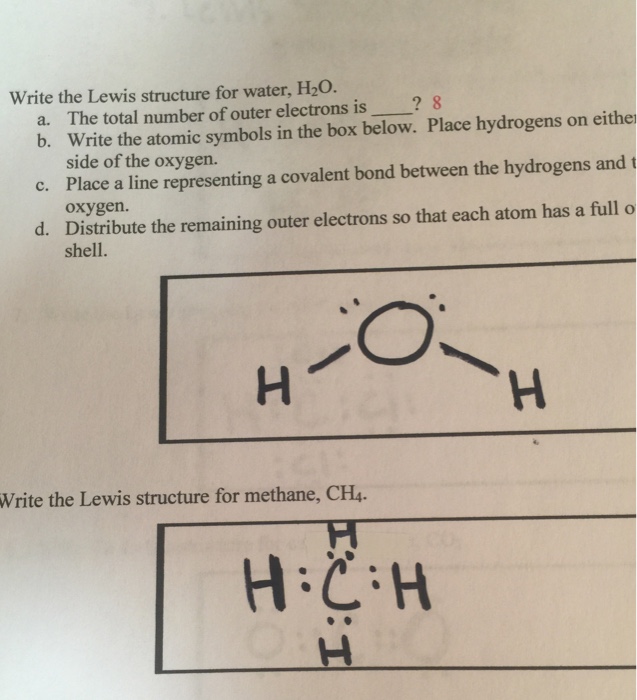
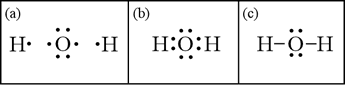
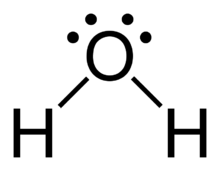
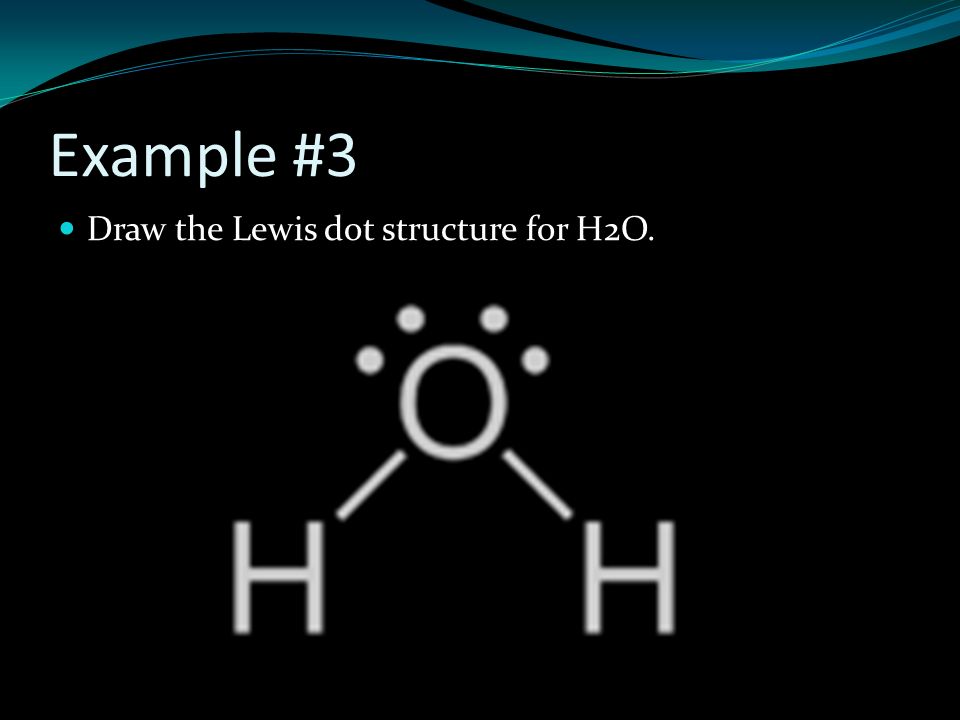
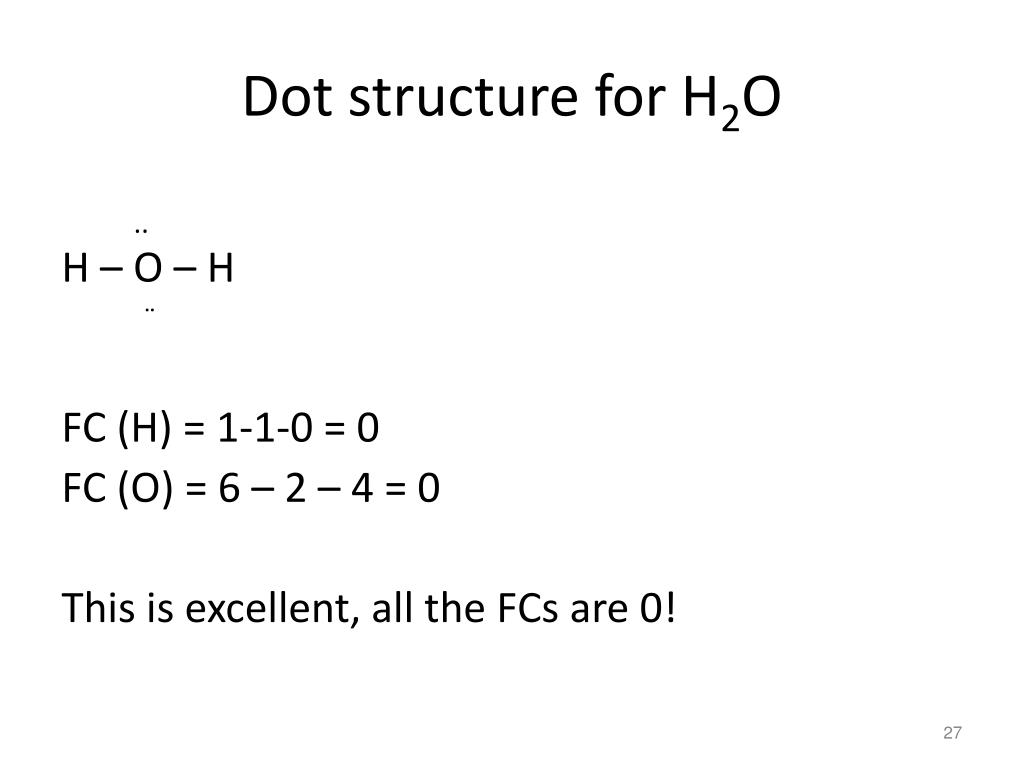
What is the Lewis dot structure for h2o? The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. You have eight valence electrons in your trial structure, so it has the correct number of electrons.

Dot diagram for h2o
1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell. Lewis Dot Diagram H2o. You can find a procedure for drawing Lewis structures at this location. You have eight valence electrons in your trial structure, so it has the. Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in. The arrangement of valance electrons in ... I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle.
Dot diagram for h2o. Lewis Structure of H 2 O (Water) - Drawing Steps. Lewis structure of water molecule contains two single bonds around oxygen atom. number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. Each step of drawing lewis structure of H 2 O are explained in this tutorial. H 2 O Lewis Structure Video. Video Transcript: Here, we're going to do a dot structure for water, H2O. Let's write that down: H2O. What we want to find out first is how many valence electrons does water have. I'm counting all the outer shell electrons. I'll need my periodic table. Answer (1 of 2): You have got three atoms, first choose which one will be the central, and that is Oxygen. Now draw separately the atomic diagram of each atom so that you would know that how many valence electrons are in each one of them. Now you know that Oxygen has 6 electrons in its valence ... Water, or H2O H 2 O, has the electron dot structure shown below. The structure must have a total of 8 valence electrons because there are 2. Click to see full answer. Furthermore, what is the electron dot structure of h2o? Drawing the Lewis Structure for H 2 O Another straight forward Lewis structure.
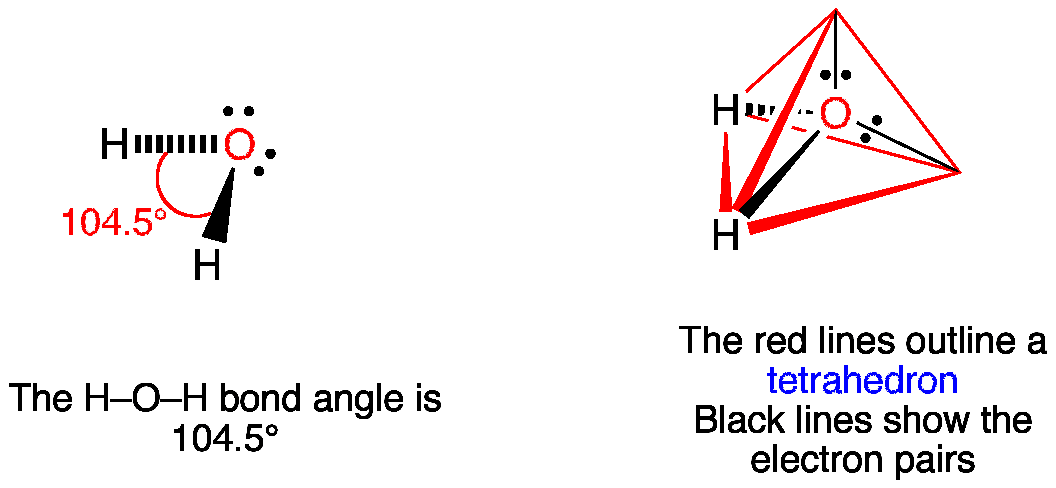
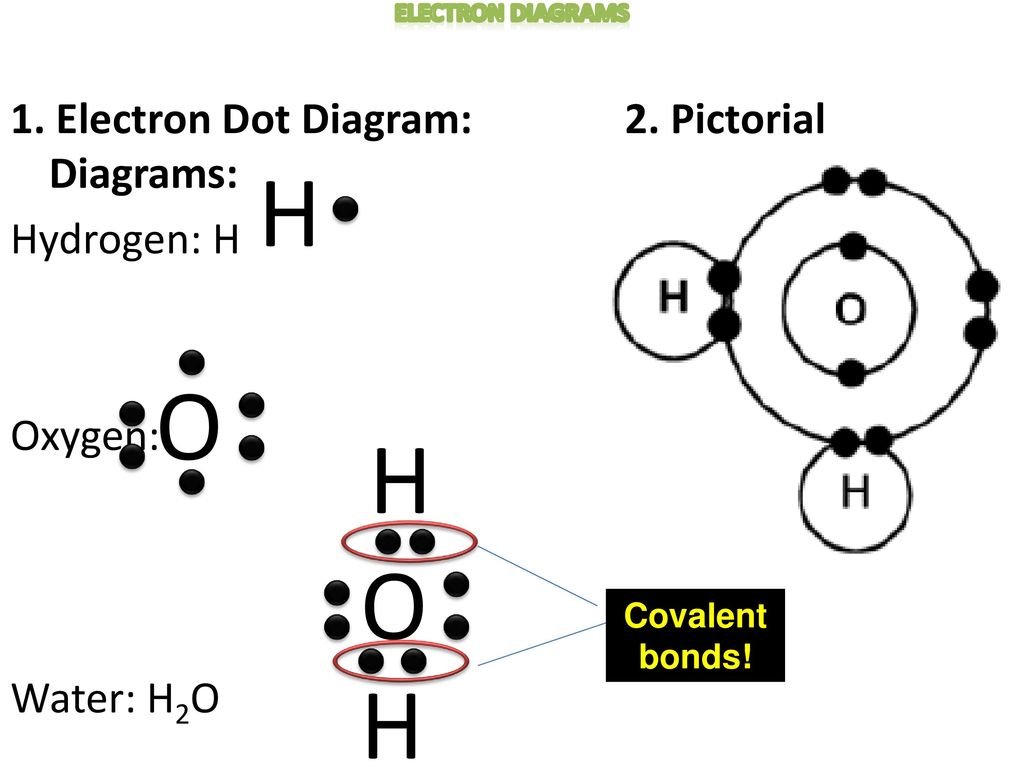
12+ Electron Dot Structure Of H2O. Electrons of the covalent bond by two dots. In order to eject an electron from a metal, a photon of a certain minimum energy must strike the sur. There are two hydrogens that bind with 1 electron from those 6, so there are 2 electrons that are binding with hydrogen. Know how and when to incorporate double and ... The Lewis structure of the triatomic H2O molecule shows two single sigma bonds between the oxygen atom and the hydrogen atoms. Moreover, these bonds leave two lone pairs of electrons on the oxygen atom that mainly contributes to the tetrahedral bent geometrical structure of the H2O molecule. It is the reason why the bond angle that should have ... The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. In H2O molecule, the Oxygen atom forms two single sigma bonds with Hydrogen atoms. Although these two Hydrogen atoms are arranged symmetrically in the plane, the two lone pairs of electrons on the Oxygen atom push these atoms. A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc...
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc... Check Answ. This is the electron dot diagram for the F2 molecule: Using numbers, complete the following statement In the fluorine molecule, there are bonding electron pair(s) and lone electron pair(s) Check Answer Draw the electron dot diagram for the H2O molecule. Then, using numbers, complete the following statement. Let's do the Lewis structure for water: H2O. On the periodic table, Hydrogen's in group 1, it has 1 valence electron; but we have two of them, so let's multiply that by 2. And Oxygen is in group 6, sometimes called 16, so it has 6 valence electrons. So 1 times 2 is 2, plus 6; 2 plus 6 equals 8. We have a total of eight valence electrons. H2o2 Dot Diagram. The chemical name for H2 O2 is hydrogen peroxide. Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in. Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the.
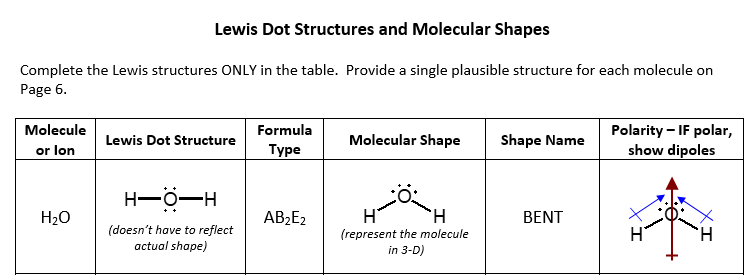
H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure.This "bent" molecular structure gives it many unique properties such as being polar.One of the most fascinating phenomena is the idea of "hydrogen bonding ...
This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have. These electron pairs form electron 'clouds' that are spread out approximately This is the reason for water's bent structure. or H2O but is better represented as H2O or even H2O giving ...
I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle.
Lewis Dot Diagram H2o. You can find a procedure for drawing Lewis structures at this location. You have eight valence electrons in your trial structure, so it has the. Firstly you need to know the number of electrons present in outermost shell of O and H atom O: 1s2 2s2 2p4 There are six electrons in. The arrangement of valance electrons in ...
1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.











0 Response to "37 dot diagram for h2o"
Post a Comment