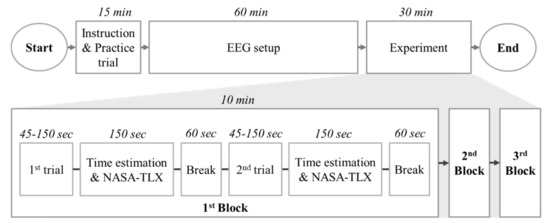
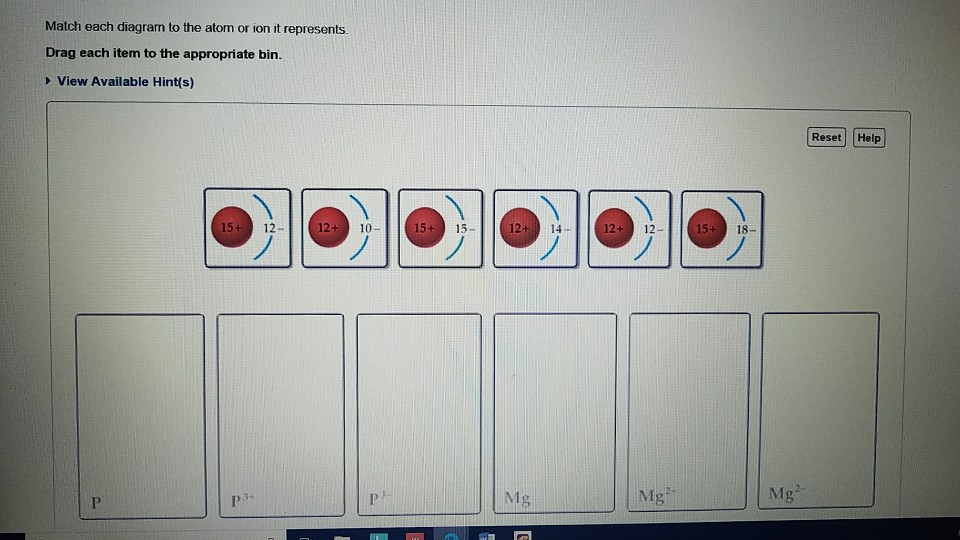
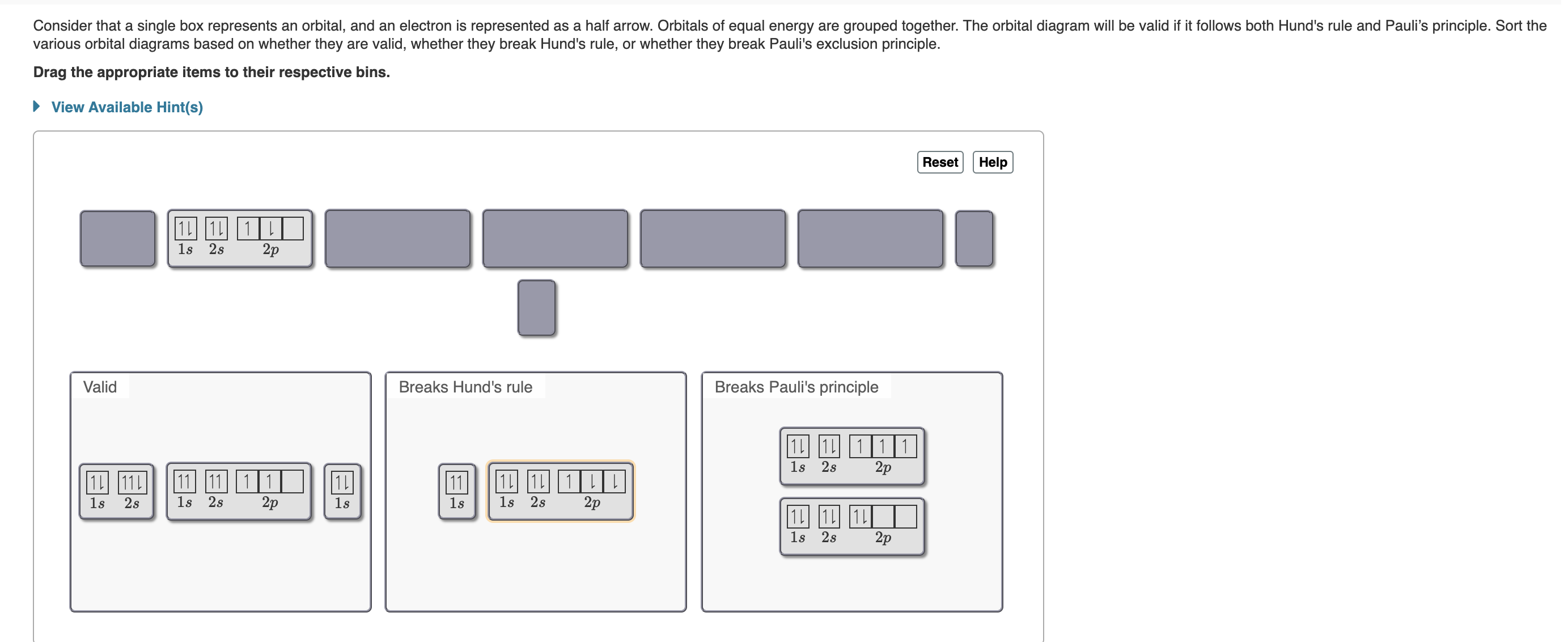
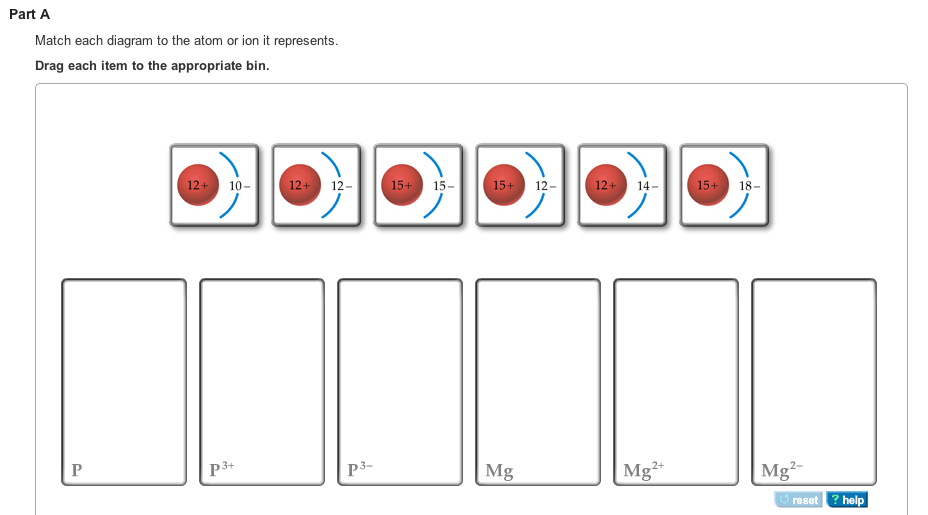
38 match each diagram to the atom or ion it represents. drag each item to the appropriate bin.
Google's free service instantly translates words, phrases, and web pages between English and over 100 other languages. Mastering Chemistry: Chapter 2 Assignment. What is the ratio of hydrogen atoms (H) to oxygen atoms (O) in 2 L of water? Enter the simplest whole number ratio in order of hydrogen to oxygen, respectively. Express your answer as two integers, separated by a comma (e.g., 3,4).
hybrid orbitals from each carbon atom. The two π bonds of the triple bond are formed from parallel overlap of the two unhybridized p atomic orbitals from each carbon. 15. Ethane, C2H6, has 2(4) + 6(1) = 14 valence electrons. The Lewis structure is: The carbon atoms are sp3 hybridized. The six C‒H sigma bonds are formed from overlap of

Match each diagram to the atom or ion it represents. drag each item to the appropriate bin.
Ions Video Lessons. Concept: Concept: Example: Problem: Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. The average adult brain represents about \(2\%\) of our body's weight, but uses \(25\%\) of the glucose in the body. Moreover, specific areas of the brain use glucose at different rates. If you are concentrating hard, (taking a test, for example) certain parts of the brain need a lot of extra glucose while other parts of the brain only use ... remember atom valence Determine number of available valence electrons: add an electron for each negative charge, remove an electron for each positive charge Draw all single covalent bonds and lone pairs: give as many atoms as possible full octets, assigning lone pairs to most electronegative atoms
Match each diagram to the atom or ion it represents. drag each item to the appropriate bin.. Write the symbol for each of the following ions: (a) the ion with a 1+ charge, atomic number 55, and mass number 133 (b) the ion with 54 electrons, 53 protons, and 74 neutrons (c) the ion with atomic number 15, mass number 31, and a 3− charge (d) the ion with 24 electrons, 30 neutrons, and a 3+ charge. Write the symbol for each of the ... Part a match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. Drag each item to the appropriate bin. Based on the data gathered in the rutherfords scattering experiments the concept of atomic structure was modified. Match each of the. So4 1 blue sphere. Match each ion with the noble gas whose electron arrangement it shares. ... The subscripts in the formula represent the number of positive and negative ions that give an overall charge of zero. Consider tin(IV) sulfide. ... Drag each item to the appropriate bin. 1 atom: monosulfide 2 atoms: dinitrogen dioxide 3 atoms: tribromide triphosphorus Which of the following are valid ionic Lewis structures? Drag each item to the appropriate bin. Which nonmetals could form an ionic compound with magnesium with the formula MgX2 (where X represents the nonmetal)? Drag each item to the appropriate bin.
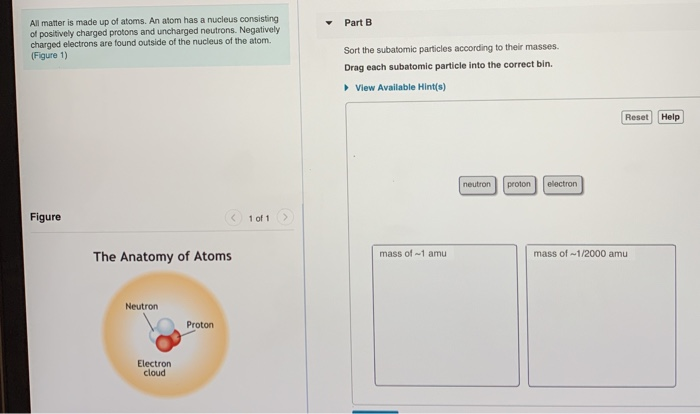
Components and Structure of the Atom. The atom consists of three types of subatomic particles: protons, neutrons, and electrons. The electron is by far the lightest of the three, while the much heavier proton and neutron have masses very similar to each other. Two of the types of particles carry an electrical charge, while the third is neutral. If we were the actually break down the above diagram into groups rather than the blocks we have, it would show how exactly how many electrons each element has. For example, the element of hydrogen, located in the uppermost left-hand corner of the periodic table, is described as 1 s 1 , with the s describing which orbital contains electrons and ... Drag each item to the appropriate bin. View Available Hint(s) Reset Help OH CH3CH2-N CH3CH2 CH3CH2C-NCH3 Н CH,C- N 1 CH CH CH CH COCH CH3CCH2CH3 CH,CH.CH Amine Amide Neither amine nor amide Part B Classify each molecule as an aldehyde, ketone, or neither. Drag each image to the appropriate location in the sequence. High Blood glucose levels Blood glucose becomes high --> Pancreas releases insulin -->Insulin binds to receptors on target cells --> cells take in glucose --> Blood glucose returns to normal 6. Drag the appropriate labels to their respective targets. (from left to right)
The atom is neutral when there are equal numbers of protons and electrons. Ions are formed when there are unequal numbers of protons and electrons in an atom. A positive ion is called a cation whereas a negative ion is called an anion. Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. Match Each Diagram to the atom or Ion It Represents. Drag Each Item to the Appropriate Bin. mastering chemistry chapter 2 assignment flashcards match each element to its period drag match each diagram to the atom or ion it represents drag each item drag each item to the appropriate bin solved match each diagram to the atom ion it represent answer to match each diagram to the atom or ion it ... Drag each tile to the correct box. Match the given symbol or molecular formula to the term that best describes it. element - organic compound - inorganic elemental molecule - inorganic compound - answers given: so2, k, CI2, c6h6 Drag each item to the appropriate bin. Match each diagram to the atom or ion it represents drag each item to the appropriate bin. For each of the following ions indicate the numbe. Which statements about subatomic particles are fal. The atomic mass of bromine is found to be 7990 amu.
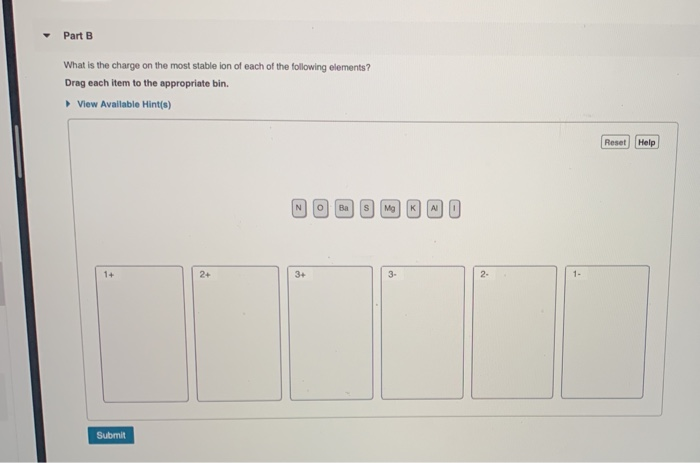
Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. You did not open hints for this part. ANSWER: Part B What is the charge on the most stable ion of each of the following elements? Drag each item to the appropriate bin. You did not open hints for this part.
Close × ☰ Click here to view the transcript.
plausible sequence. Make sure you draw the correct structure for each intemediate product and clearly indicate the reagent(s) required for each reaction. The following list of suggested reagents is sufficient to accomplish all necessary reactions, but you may use other reagents if you wish. One of the intermediates is shown for you. Marks 8
We can draw N 2 O resonance structures to identify the most stable structure of N 2 O. Oxidation numbers of nitrogen in N 2 O is decided from most stable structures. Finally we build the shape of N 2 O molecule.. N 2 O resonance structures. Electronegativity of oxygen is higher than nitrogen. This means a negative charge on oxygen atom is more stable than a negative charge on nitrogen atom.
Chapter 7 7.2 Effective Nuclear Charge 7.3 Size of Atoms & Ions 7.4 Ionization 7.5 Electron Affinity
This photo about: Match Each Diagram to the atom or Ion It Represents. Drag Each Item to the Appropriate Bin., entitled as Chemistry Archive November 17 2016 Match Each Diagram To The Atom Or Ion It Represents. Drag Each Item To The Appropriate Bin. - also describes Chemistry Archive November 17 2016 and labeled as: match each fitness skill to its definition,match each karate,match each public ...
A positive ion is called a cation whereas a negative ion is called an anion. Part A. Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. An atom is made up of a positively-charged nucleus surrounded by negatively-charged electrons. The atom is neutral when there are equal numbers of protons and electrons.
Labeling, ranking, sorting, or sentence completion questions. All of these question types require you to position items into an area of the answer box. Answer these kinds of questions on a computer, not on a smartphone. Press Tab to move forward or Shift/Tab to move backwards through the provided answer items.
Very important for protein structure!! Remember that the pKa = pH when ½ of an available amount of an ionizable group is ionized. - Let's take a look at the titration curve for Alanine - Looks very much like what we saw for acetic acid last time except that it has 2 midpoints (pKa's) - one for each proton α-COOH and α-NH 3 +
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is sometimes used to indicate a shared pair of electrons: A single shared pair of electrons is called a single bond.
Chemistry questions and answers. Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin View Available Hint (s) Reset Help 15+ 12- 12+10- 15+15- 1214 12+ 12- 1518- 2- Mg Mg Mg. Question: Match each diagram to the atom or ion it represents.
remember atom valence Determine number of available valence electrons: add an electron for each negative charge, remove an electron for each positive charge Draw all single covalent bonds and lone pairs: give as many atoms as possible full octets, assigning lone pairs to most electronegative atoms
The average adult brain represents about \(2\%\) of our body's weight, but uses \(25\%\) of the glucose in the body. Moreover, specific areas of the brain use glucose at different rates. If you are concentrating hard, (taking a test, for example) certain parts of the brain need a lot of extra glucose while other parts of the brain only use ...
Ions Video Lessons. Concept: Concept: Example: Problem: Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin.
























0 Response to "38 match each diagram to the atom or ion it represents. drag each item to the appropriate bin."
Post a Comment