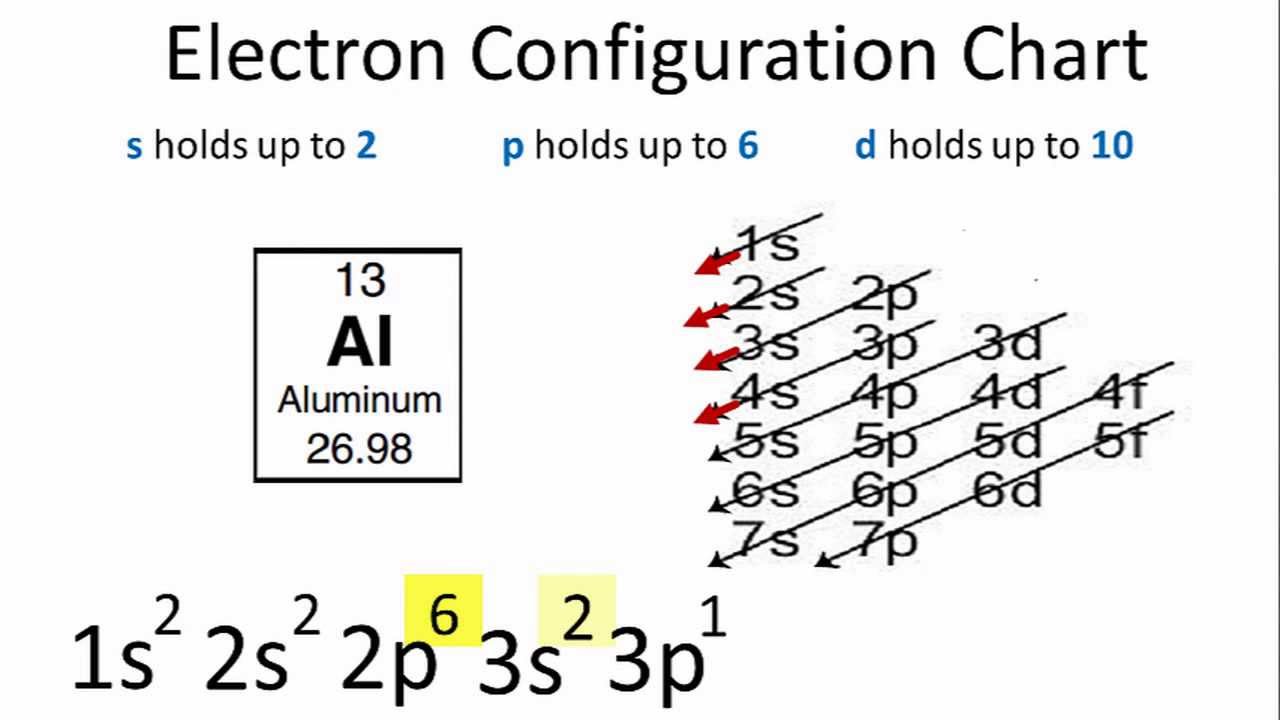
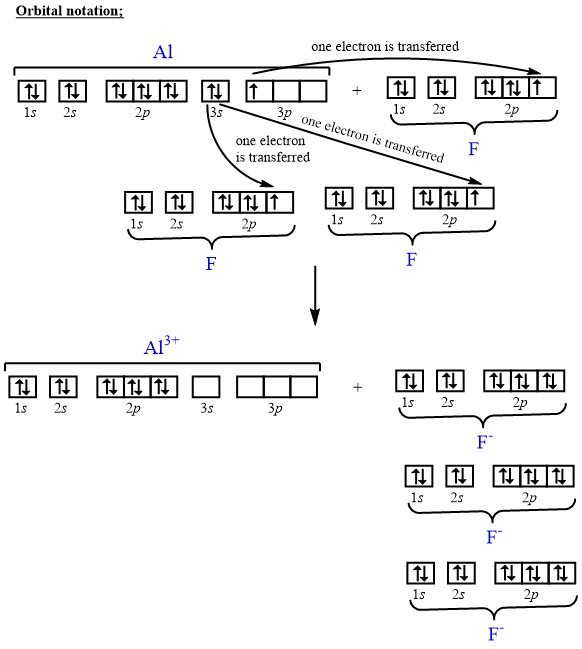
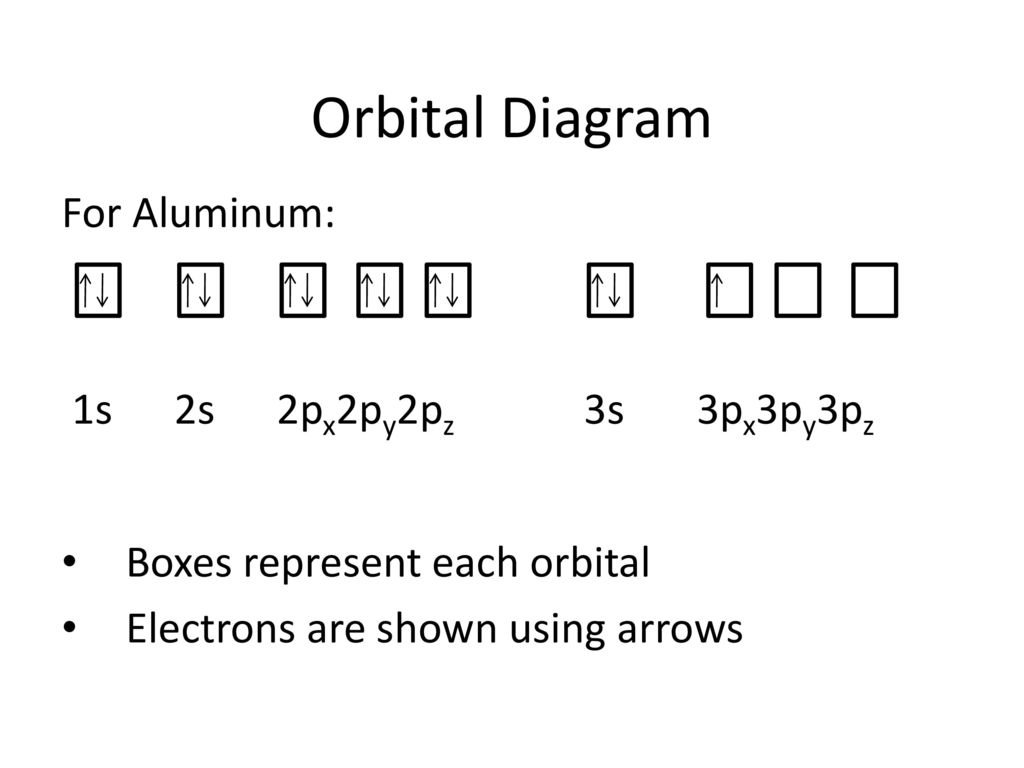
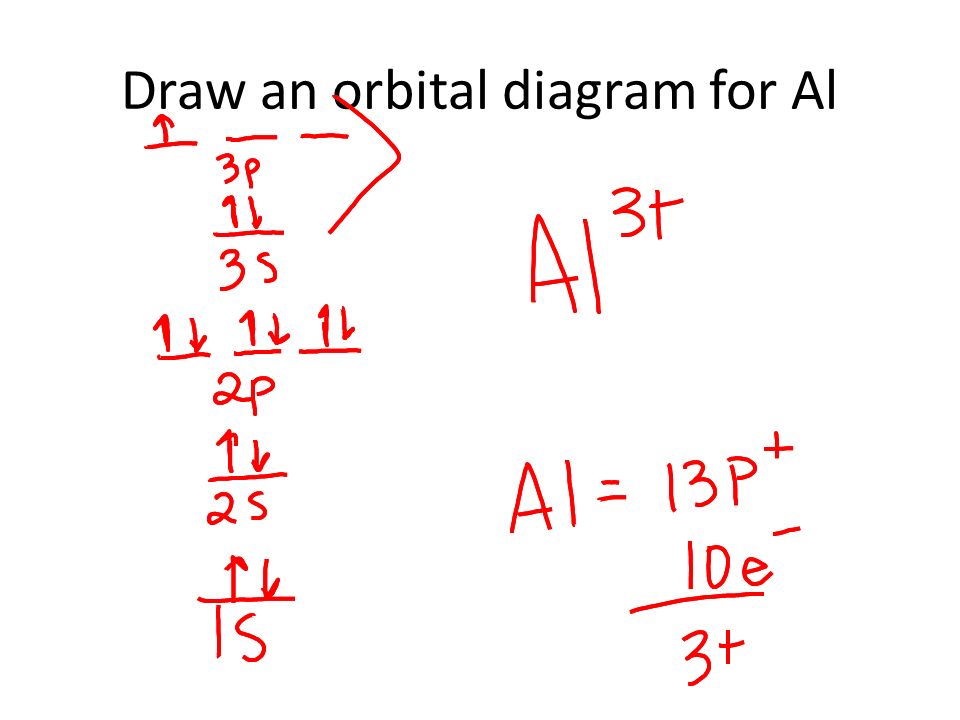
38 orbital diagram of aluminum
10 Jan 2020 — Apply the rules and principles of electron configuration to draw the orbital diagram of aluminum. Use the periodic table to help you.1 answer · Top answer: The electronic configuration of Aluminum is 2,8,3.Explanation:Aluminum is a metallic element belonging to group 3 of periodic table. Its atomic number ...
Aluminium (Al) has an atomic mass of 13. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
A geostationary orbit, also referred to as a geosynchronous equatorial orbit (GEO), is a circular geosynchronous orbit 35,786 kilometres (22,236 miles) in altitude above Earth's Equator (42,164 kilometers in radius from Earth's center) and following the direction of Earth's rotation.. An object in such an orbit has an orbital period equal to Earth's rotational period, one sidereal day, and so ...

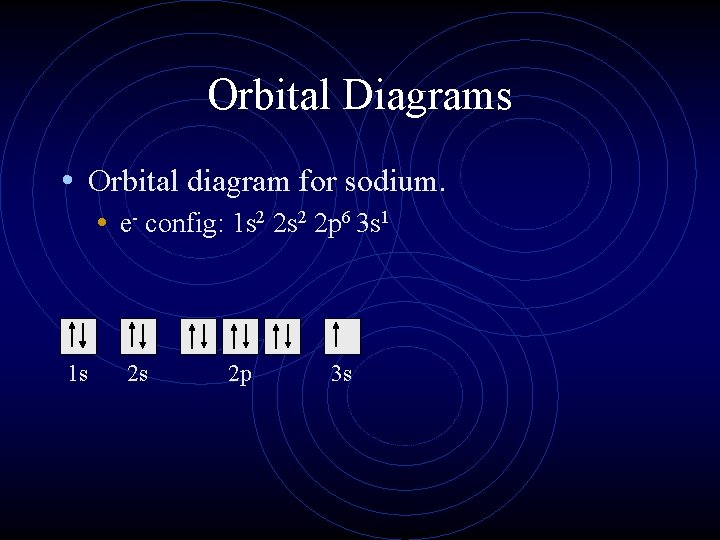
Orbital diagram of aluminum
Electronic configuration of the Aluminum atom. Valence electrons. Orbital diagram.
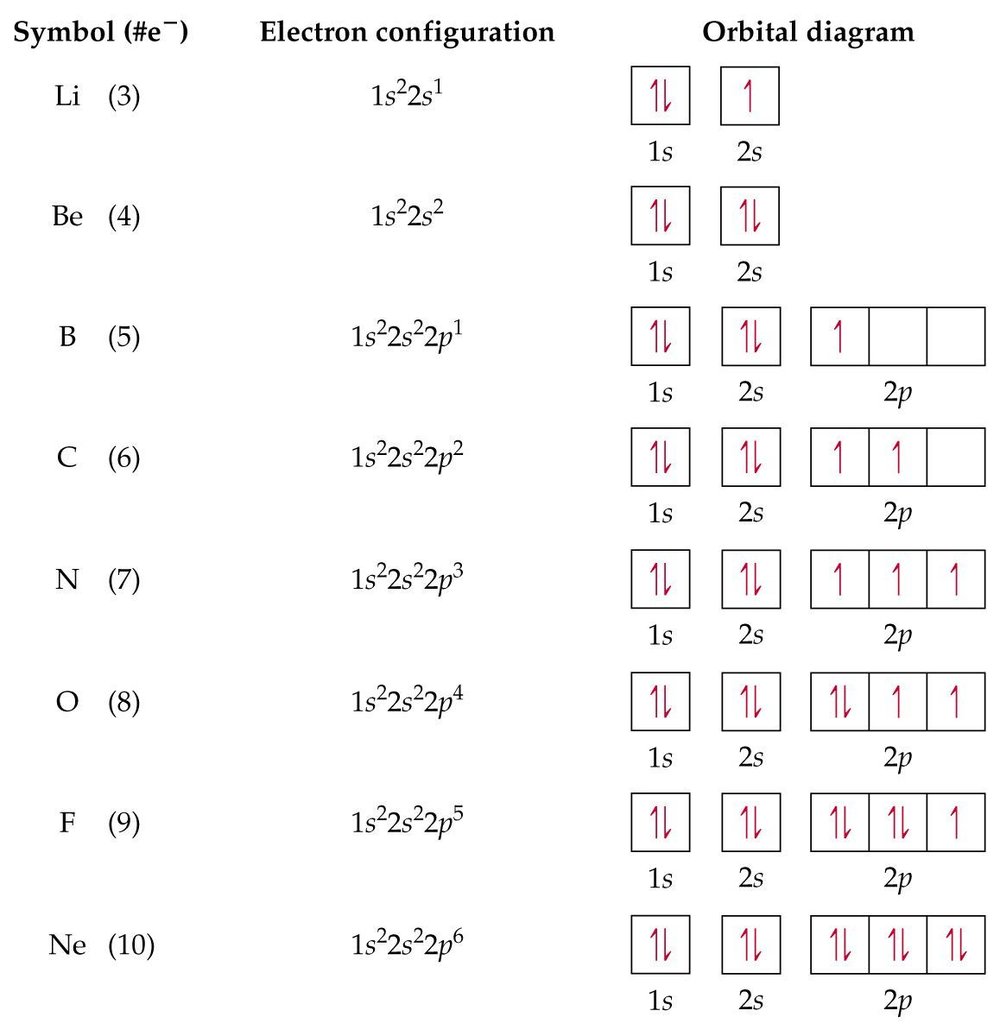
Hydrogen(H) Electron Configuration with Full Orbital Diagram. Hydrogen electron configuration is 1s 1. Hydrogen is a s-block element. This article gives an idea about the electron configuration of hydrogen, period and groups, valency and valence electrons of hydrogen, bond formation, compound formation, application of different principles. The first element of the periodic table is hydrogen ...
Gabbard diagram of almost 300 pieces of debris from the disintegration of the five-month-old third stage of the Chinese Long March 4 booster on 11 March 2000. The trackers (NORAD) who fed the database were aware of other objects in orbit, many of which were the result of in-orbit explosions. Some were deliberately caused during the 1960s anti-satellite weapon (ASAT) testing, and others were ...
Orbital diagram of aluminum.
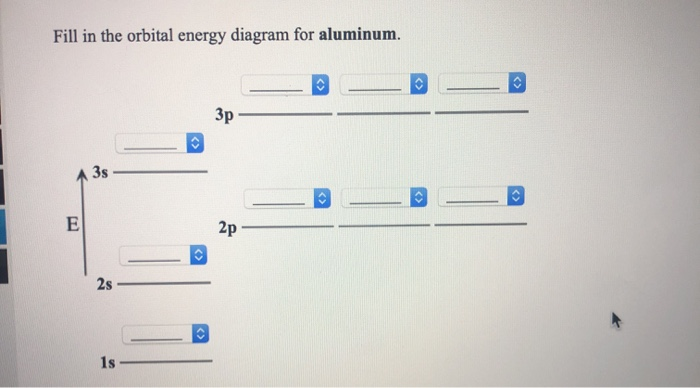
14 Aug 2020 — Electron configurations and orbital diagrams can be determined by ... Aluminum (atomic number 13), with 13 electrons and the electron ...
20 Nov 2020 · 1 answerAluminum (Al) has only atomic orbitals. As a 3rd row element, however, it has a complete Ne electron configuration. One way to denote this is to write it as ...How many electrons are in the highest energy level of ...2 answers6 Aug 2021What is the orbital diagram for magnesium?1 answer20 Aug 2015More results from www.quora.com
28/11/2021 · The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of beryllium atom.
09/12/2021 · diagram worksheet 1 – Classify matter as a pure substance (element or compound) or a mixture based on a chemical formula or a particle diagram. Over 100 existing elements are listed and classified on the. Part 3: Match each diagram with its correct description. Diagrams will be used once. We will classify matter as pure substances or mixtures by using a variety of diagrams. I will ...
The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move ...24 Oct 2016 · Uploaded by Wayne Breslyn
Oxygen(O) electron configuration with full orbital diagram. Oxygen(O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is ‘O’. The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency ...
Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne] 3 ... What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1 s, 2s, 2p, 3s, 3p, 4s ...
26 Jan 2021 — Here we have covered the Aluminium Electron Configuration with the symbol of Aluminium. The Orbital Diagram of Aluminium also given here.
A Molecular Orbital Diagram for a diatomic molecule (two atoms) varies in the number of electrons. How do you populate the electrons? Answer • Count the valence electrons on the molecule. That's the number of valence electrons on each atom, adjusted for any charge on the molecule. (eg C 2 2-has 10 valence electrons: 4 from each carbon -- that's 8 -- and two more for the 2- charge). • Fill ...
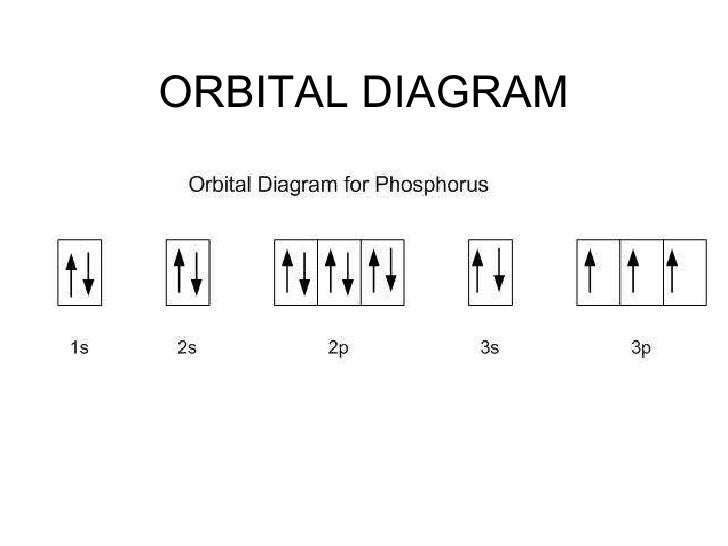
The order is summarized under the diagram. ... aluminum: 13: 1s 2 2s 2 2p 6 3s 2 3p 1: silicon: 14: 1s 2 2s 2 2p 6 3s 2 3p 2: phosphorus: 15: 1s 2 2s 2 2p 6 3s 2 3p 3: sulfur: 16: 1s 2 2s 2 2p 6 3s 2 3p 4: chlorine : 17: 1s 2 2s 2 2p 6 3s 2 3p 5: argon: 18: 1s 2 2s 2 2p 6 3s 2 3p 6: A. Box Diagrams of Electron Configuration If an atom has a partially filled sublevel, it may be important to ...
1 answerWe are asked to give the full orbital diagram for aluminum (Al). Aluminum has 13 electrons to distribute because its atomic number is 13 and neutral atoms ...
27/11/2021 · MO Diagram of XeF4. An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. When atoms combine with other atoms to make molecules, some of the atomic orbitals adds up to form molecular orbitals which are the same in number.
























0 Response to "38 orbital diagram of aluminum"
Post a Comment