42 cu ni phase diagram
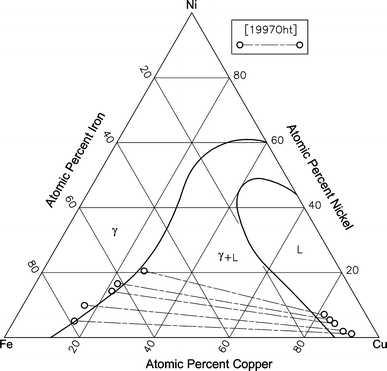
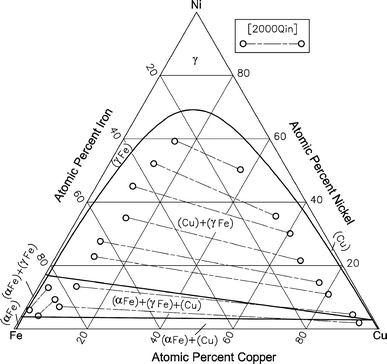
pressure to be constant at one atmosphere. Phase diagrams for materials with more than two components are complex and difficult to represent. An example of a phase diagram for a ternary alloy is shown for a fixed T and P below. Phase diagrams for binary systems ternary phase diagram of Ni-Cr-Fe
English: Cu-Ni phase diagram. Polski: Wykres fazowy Cu-Ni. Date: 28 March 2011: Source: Own work: Author: Joanna Kośmider: Permission (Reusing this file) Public domain Public domain false false: I, the copyright holder of this work, release this work into the public domain. This applies worldwide.
There is two types of important phase diagrams are used one is a slow process diagram, and another is a fast process diagram. Answer and Explanation: 1 Phase diagram of the Ni-Cu is
Cu ni phase diagram
Introduction to the Cu-Ni phase diagram relating to the use of the Lever Rule for determining composition and phase weight percentages.
We have examined isomorphous phase diagrams, and used the example of the Cu-Ni phase diagram. In this module we will examine eutectic phase diagrams. A eutectic system has two components, and they have limited solubility. Upon cooling, the liquid will transform into two mixed solid phases. We will use the Pb-Sn phase diagram as an example.
In this work, phase relations and thermal stabilities of equilibrium phases in the Cu-Ni-S system have been reviewed. The calculated phase diagram of Cu-N system has been validated. At T > 630 K, in the N-rich corner, large scatter in data has been observed and discussed in detail.
Cu ni phase diagram.
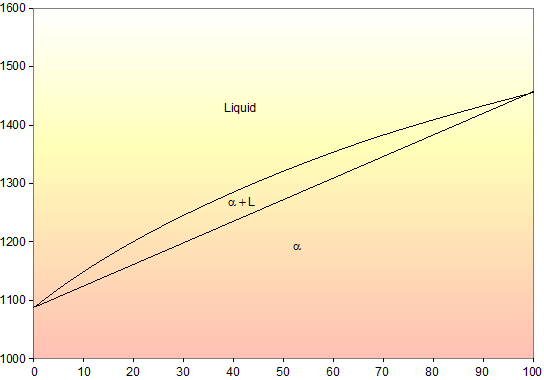
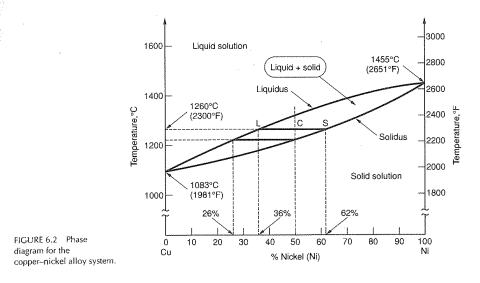
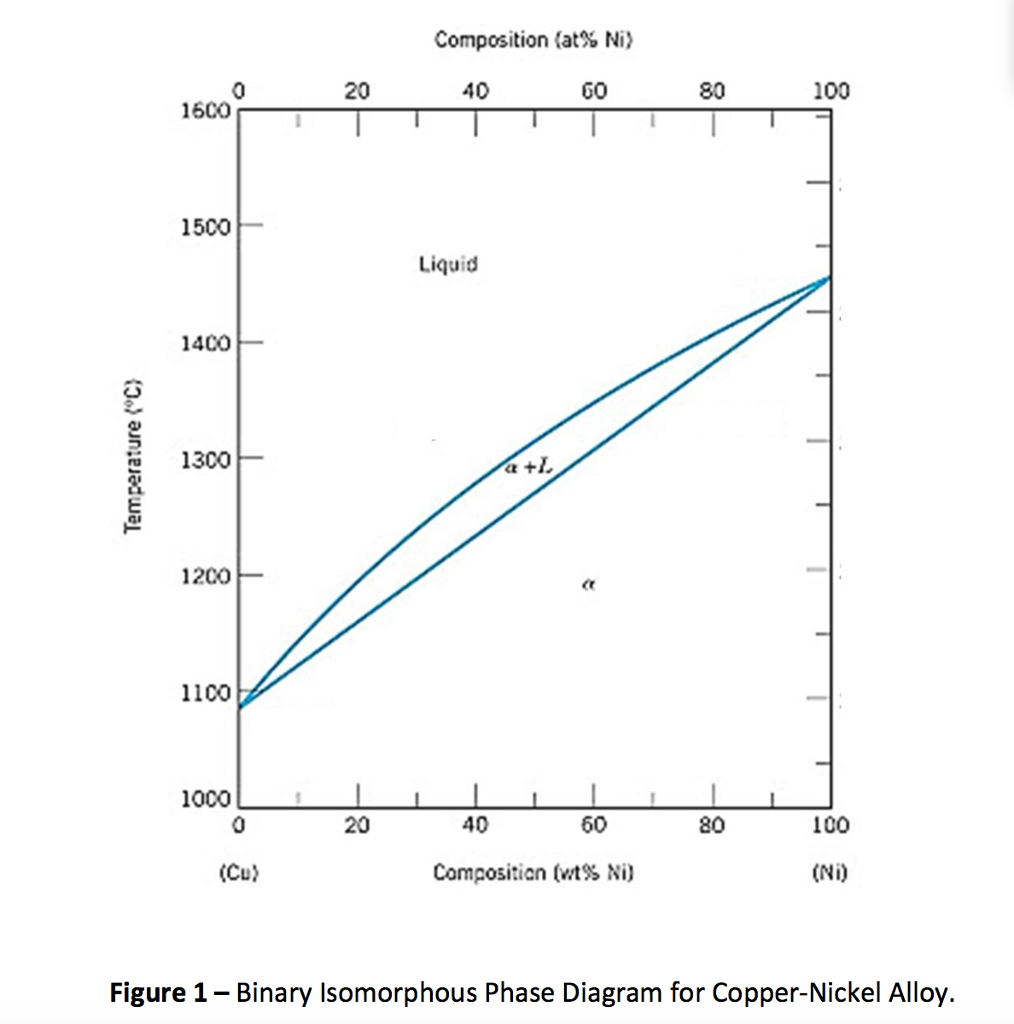
Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C.
Move the mouse over the phase diagram to view temperature and composition at any point. View micrographs for the Cu-Ni system. List all systems with phase diagrams
Al-Cu-Ni PHASE DIAGRAM This phase diagram is helpful in the analysis of 2618-type heat-resistant alloys and 339.0-type piston alloys that contain nickel, copper, and other alloying components (Tables 7.1 and 7.2). The ternary Al7Cu4Ni phase forms in the aluminum corner of the Al-Cu-Ni system.
The thermodynamic calculation of phase equilibria in the Cu-Ni-Si alloy system was carried out using the CALPHAD method. The calculations show that there are three two-phase areas and two three ...
Shown below is the Cu-Ni phase diagram (Figure 9.3a) and a vertical line constructed at a composition of 70 wt% Ni-30 wt% Cu. (a) Upon heating from 1300°C, the first liquid phase forms at the temperature at which this vertical line intersects the α-(α + L) phase boundary--i.e., about 1345°C. )-)-
Part of the phase diagram of the Cu-Ni-Mn system from 0 to 20% Ni and from 30 to 50% Mn is refined with the help of a theoretical analysis and based on the experimental data.
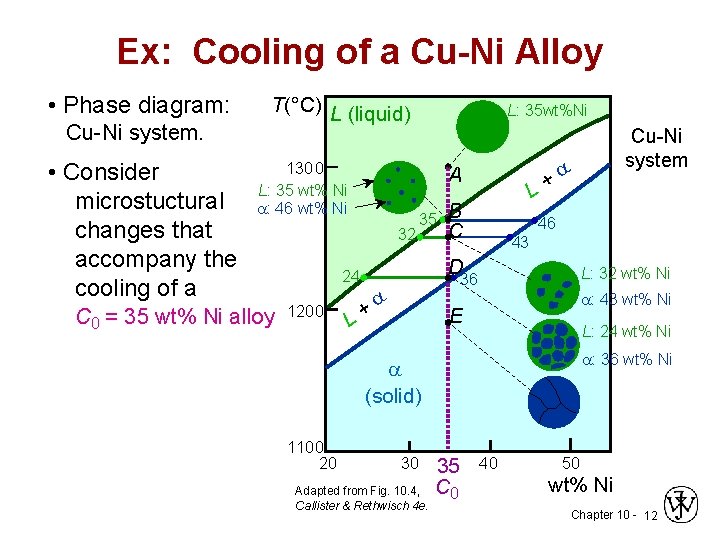
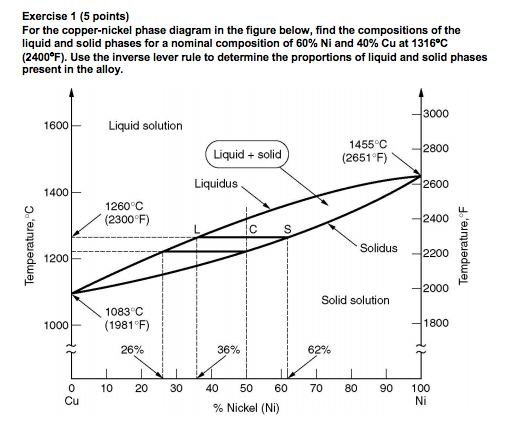
Cu-Ni phase diagram A(1100, 60): 1 phase: B(1250, 35): 2 phases: L + Determination of phase(s) present Melting points: Cu = 1085°C, Ni = 1453 °C Solidus - Temperature where alloy is completely solid. Above this line, liquefaction begins. Liquidus - Temperature where alloy is completely liquid. Below this line, solidification begins.
This video explains binary phase diagrams, specifically the Cu-Ni System. For further studies: visit https://www.doitpoms.ac.uk/tlplib/phase-diagrams/printal...
Example: Cu-Ni phase diagram (only for slow cooling conditions) Liquidus line: the line connecting Ts at which liquid starts to solidify under equilibrium conditions Solidus: the temperature at which the last of the liquid phase solidifies Between liquidus and solidus: P =2. Chapter 8 9
EN380 Homework #6 Solution 1.The binary (two-component) phase diagram for copper-nickel alloys is shown below. A Cu - Ni alloy contains 30 wt. % Cu at a temperature of 1350 C. Find the following: (a)melting point for pure copper.1084 C (b)melting point for pure nickel.1455 C (c)wt. % Niin the (entire) binary alloy.70% Ni
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
金属やセラミックスの状態図、エンリンガム図などの情報を提供しています。 一部、不確かなものもありますので、自己 ...
Cu-Ni Phase Diagram 1000 1200 1400 1600 26% 36% 50% 62% 1260 (°C) Cu Ni Liquid Solid L+S Substitutional Solid Alloys Pure Metals Pure Metals Time T Time T Time L S 4 Pb-Sn Phase Diagram Pb (lead) Sn (Tin) Temperature, °F 0 100 200 300 400 500 600 α α+L L β+L β α+β 362 °F 61.9% Sn Eutectic Composition and Temperature Time α 5 Fe-C ...
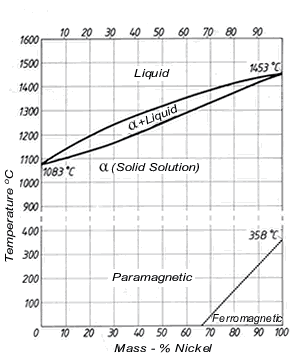
The density of copper (8.93 kg/dm 3 at 20 °C) varies only slightly with increasing nickel content (density of nickel at 20 °C = 8.9 kg/dm 3) and is 8.9 kg/dm3 for all Cu-Ni alloys specified in DIN 17 664. This aspect can also be seen in Table 7 with the physical properties of the Cu-Ni resistance alloys to DIN 17 471.
Phase diagrams 0 20 40 60 80 100 wt% Ni 1000 1100 1200 1300 1400 1500 1600 T(°C) L (liquid) α (FCC solid solution) L α s s Cu-Ni phase diagram • Rule 1: If we know T and C o, then we know:--the # and types of phases present. • Examples: Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel ...
Phase Diagrams • Indicate phases as a function of T, C, and P. • For this course:-binary systems: just 2 components.-independent variables: T and C (P = 1 atm is almost always used). Phase Diagram for Cu-Ni system Fig. 11.3(a), Callister & Rethwisch 9e. (Adapted from Phase Diagrams of Binary Nickel Alloys, P. Nash, Editor, 1991. Reprinted
Section-Editor: Hiroaki Okamoto (Phase Diagrams) Cite this content Pierre Villars (Chief Editor), PAULING FILE in: Inorganic Solid Phases, SpringerMaterials (online database), Springer, Heidelberg (ed.) SpringerMaterials Cu-Ni Binary Phase Diagram 0-24 at.%
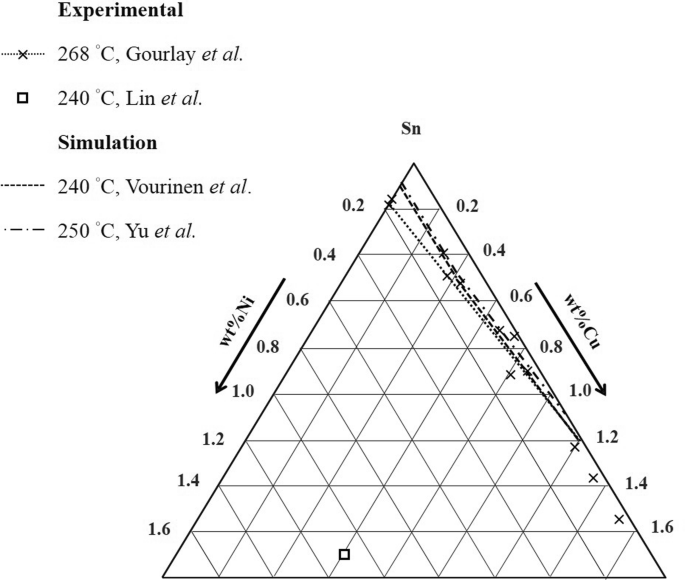
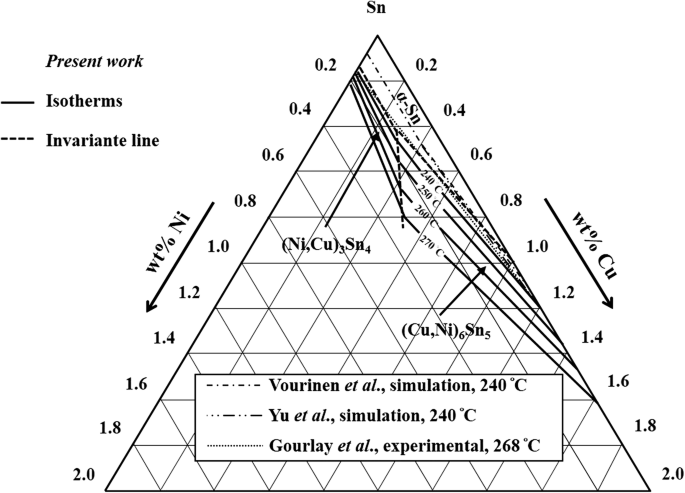
To generate a new Sn-Cu-Ni phase diagram based on reliable experimental data including four liquidus lines at 240 °C, 250 °C, 260 °C, and 270 °C. Characterize the invariant (or invariants) line for the equilibrium between a liquid phase with two IMCs Corroboration of Attainment of Homogeneous Liquid During Fast Quenching
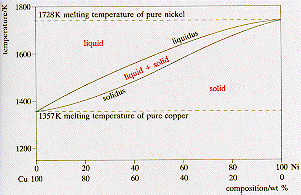
The binary phase diagram shown for the copper-nickel alloy indicates that these materials can form both liquid and solid solutions over the full range of composition from Cu to Ni. Above 1728 K, the melting point of pure Ni the alloys ar in the liquid phase. Between 1728 K and 1357 K (the melting point of Cu) the alloys can be either solid or ...
Binary phase diagram If a system consists of two components, equilibrium of phases exist is depicted by binary phase diagram. For most systems, pressure is constant, thus independently variable parameters are -temperature and composition. Two components can be either two metals (Cu and Ni), or a metal and a compound (Fe and Fe3C), or two ...
diagrams. The binary phase diagram is used for system of two components and its classifications depend on number of phases as; isomorphous contain system with two phases such as Cu - Ni system. The...
phase diagram of the system, Figure 1203.01.02. Note that, apart from the pure elements, all alloys of Cu and Ni have a range of temperatures over which solidification (freezing) occurs - the field is defined by the liquidus and solidus lines on the phase diagram. We now need to look more closely at the consequences of this.
The Cu-Ni and binary phase diagram (Figure 10.3) is the simplest type of binary phase diagrams for two metals. Figure 10.3 shows that Cu and Ni are mutually soluble at room temperature throughout the entire range of compositions. Remember from Chapter 5that we discussed the HumeRothery -






![Cu-Ni phase diagram [2]. | Download Scientific Diagram](https://www.researchgate.net/profile/Andras-Kovacs-5/publication/321224714/figure/fig1/AS:631621728337962@1527601763764/Cu-Ni-phase-diagram-2.png)





![PDF] Cu-Ni-Sn: A Key System for Lead-Free Soldering ...](https://d3i71xaburhd42.cloudfront.net/cfd17952624149800434cbd2148bfd158b8f0565/11-Figure2-1.png)










0 Response to "42 cu ni phase diagram"
Post a Comment