37 hcn molecular orbital diagram
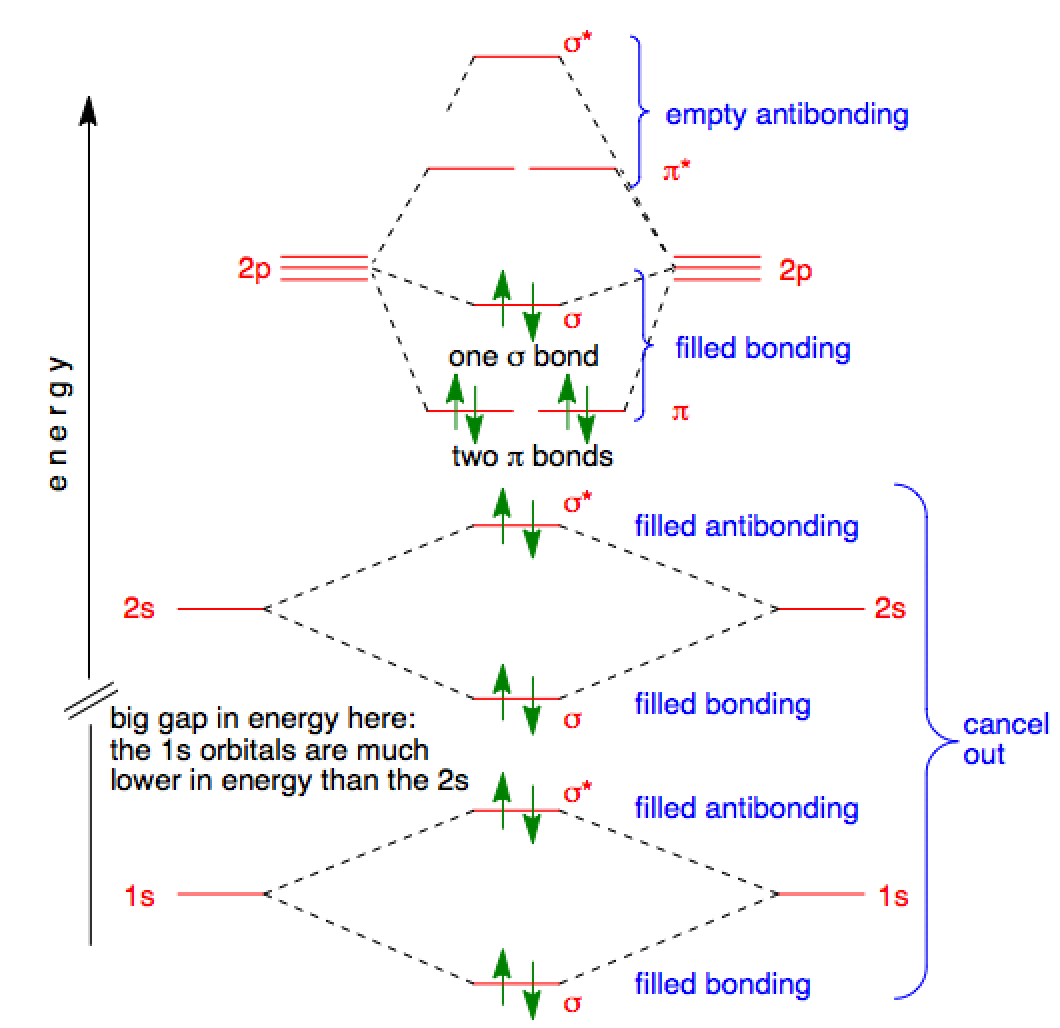
Jul 23, 2021 · Once we know the Lewis structure and Molecular Geometry of any molecule, it is easy to determine its bond angles and polarity. As this molecule has a linear molecular geometry, HCN has bond angles of 180 degrees. HCN Shape. As both Hydrogen and Nitrogen are placed far from each other at bond angles of 180 degrees, it forms a linear shape. HCN Polarity 2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2.
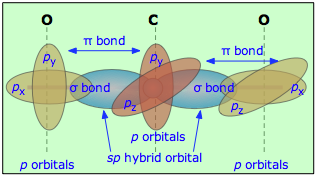
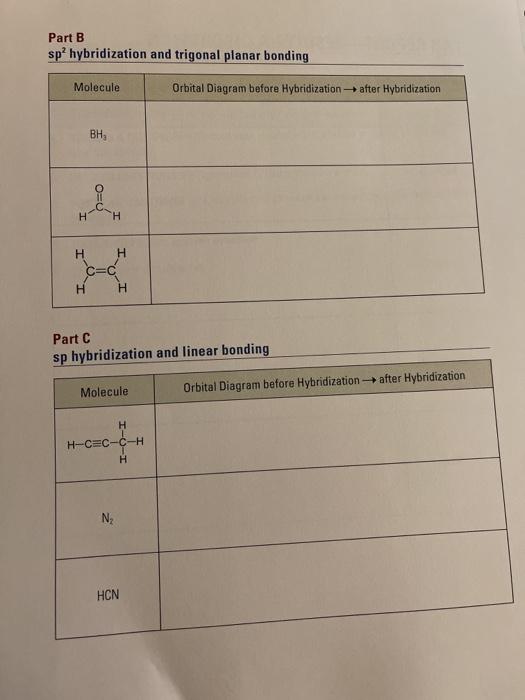
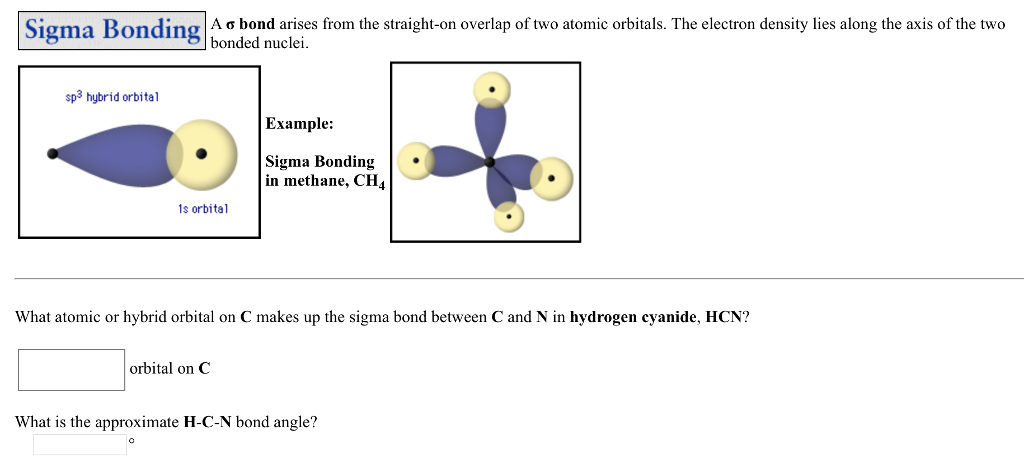
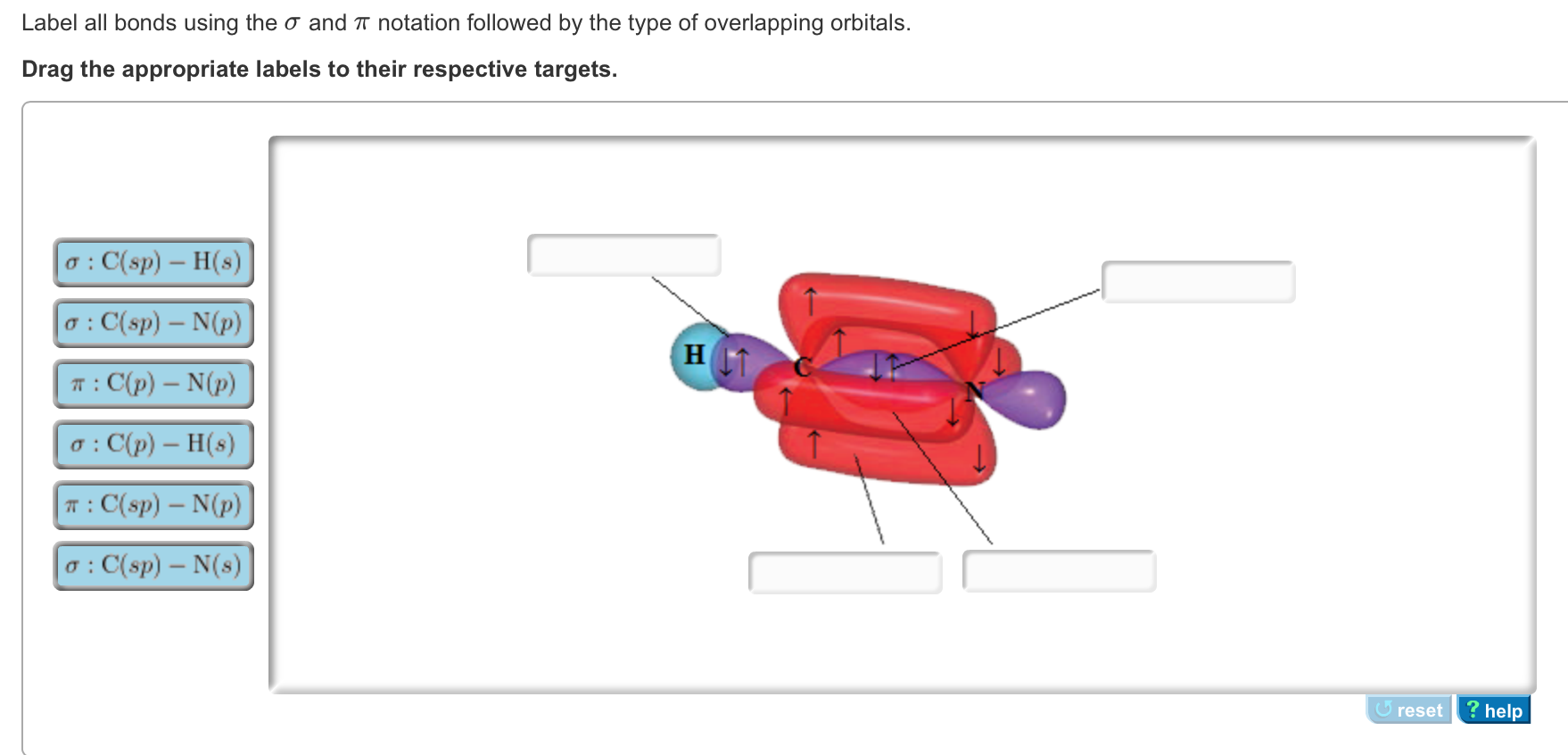
the bonding between the carban and the nitrogen in hydrogen cyanide or hydrocyanic acid is a triple bond, hence the hybrid orbital is sp, due to the linear geometry of the molecule
Hcn molecular orbital diagram
Mar 25, 2021 · Generally, molecules with linear molecular geometry have sp hybridization as the central atom forms bonds with two atoms only. Number of sigma bonds in the molecule. Another way to find out the hybridization of the molecule is to look at the number of sigma bonds formed. In HCN, there are two sigma bonds, C-H and C-N. The number of sigma bonds is equal to the number of hybrid orbitals formed. Nov 08, · RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call (sigma, pi) and place the obtainable electrons interior the proper atomic orbitals and molecular wiringall.com: Resolved. Steps in predicting the hybrid orbitals used by an atom in bonding: 1. Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion).
Hcn molecular orbital diagram. Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. There are two sigma bonds in HCN: C-H and C-N. The number of sigma bonds created equals the number of hybrid orbitals. As a result, the hybridization for the HCN molecule is sp hybridization. Molecular Geometry. The liner molecular geometry of hydrogen cyanide has bond angles of 180 degrees. Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 21) The orbital hybridization on the carbon atom in HCN is. A) sp. B) sp2. C) sp3. D) none of the above. 22) What orbital hybridization is expected for the central atom in a molecule with a trigonal planar geometry?
In the HCN molecule, C atom includes sp - hybridized orbital, since it will combine with only two other atoms to form HCN. One of the sp - hybrid orbitals of Carbon atom overlaps with 1 s orbital of H atom, while other sp - hybrid orabital mixes with one of Nitrogen's atom's three atomic p orbitals which were unhybridized. For the molecule hydrogen cyanide (HCN):a) Draw the filled atomic orbitals of the nitrogen atom.b) Which atomic orbitals are used for hybridization and event... RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call. Create a molecular orbital diagram of the linear BeF2 molecule. For Be use a basis set that consists of the 2s, 2px, 2py, 2pz atomic orbitals. Unlike in question 4. Jan 15, 2022 · In the case of HCN, let us look at how the atomic orbitals fuse to make molecular orbitals. Electronic configuration of C is 2s2 2p2, electronic configuration of H is 1s1, and electronic configuration of N is 2s2 2p3. Here, one sp orbital of C fuses with 1s orbital of H. And the other sp orbital of C fuses with one of the p orbitals of Nitrogen.
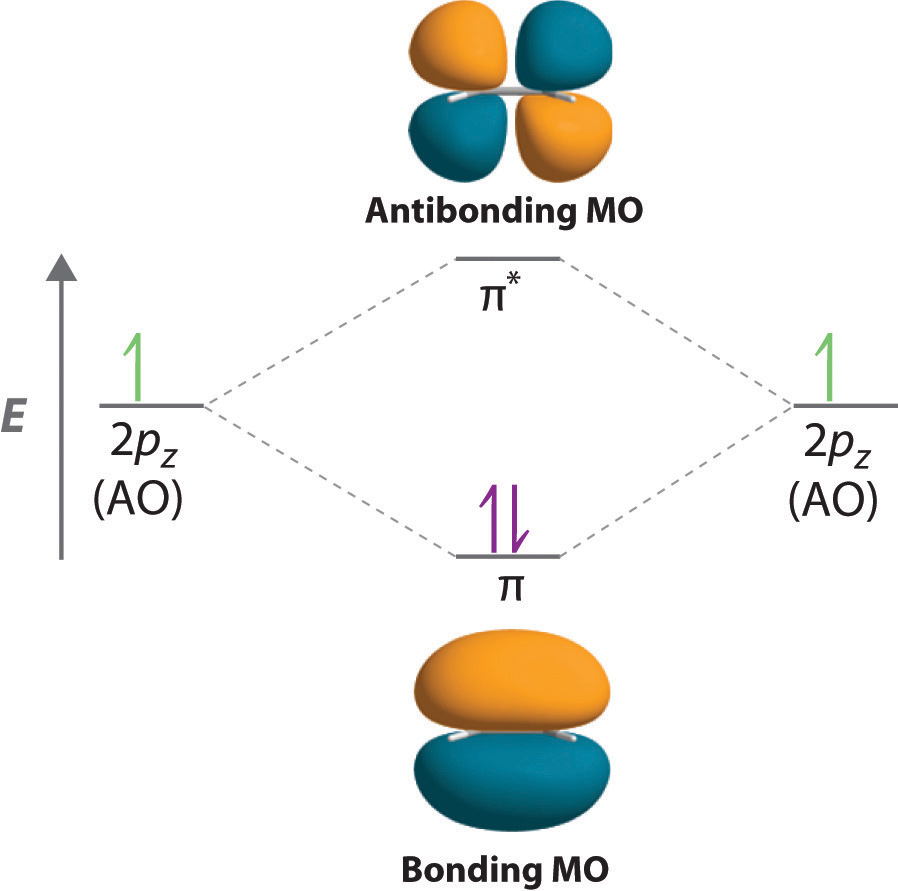
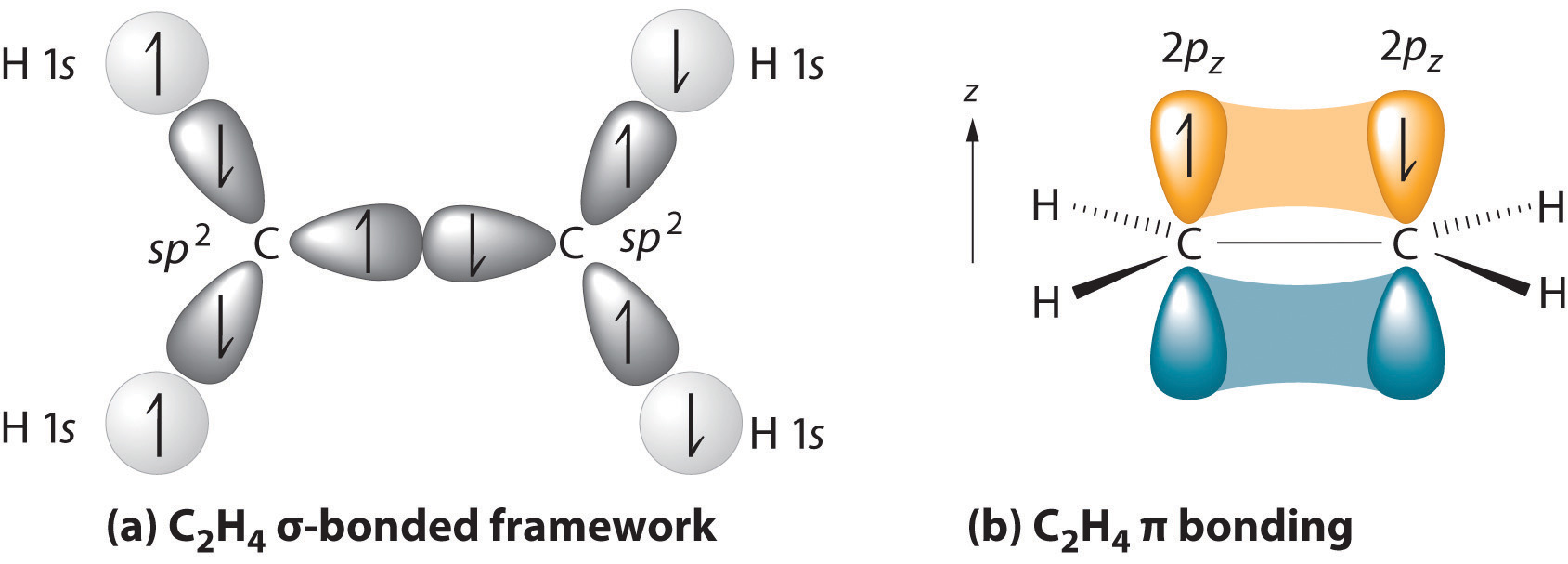
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN). Describe HCN molecular bond by using Valence Bond Theory. Because px orbital of C and N will form sigma bond, this leaves with two N atom p-orbitals which form two mutually perpendicular pi bonds to the two atomic p orbitals on the C atom. HCN thus has one single and one triple bond. Click to see full answer. How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s Here, s, p, and d are atomic orbitals and z is the bond axis. Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.
Molecular Geometry. Hydrogen cyanide has linear molecular geometry with bond angles of 180 degrees. As hydrogen and nitrogen tend to be far from each other, HCN forms a linear shape. It is slightly polar as nitrogen tries to pull the electrons to itself due to its electronegative value.
Cyanide Molecular Orbital Diagram. MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in ...
Hydrogen cyanide is a one-carbon compound consisting of a methine group triple bonded to a nitrogen atom It has a role as a human metabolite, an Escherichia coli metabolite and a poison.It is a hydracid and a one-carbon compound.It is a conjugate acid of a cyanide.It is a tautomer of a hydrogen isocyanide.

This photograph depicted Enteric Diseases Laboratory Branch (EDLB), Public Health scientists, as they were preparing enteric bacteria samples for “DNA fingerprintingâ€, using pulsed-field gel electrophoresis (PFGE).
Answer (1 of 7): Summing up the number of σ -bond formed by the desired atom (here N) and the number of lone pair on it we can easily know the hybridization of it. If the sum is 2 →hybridization−sp If the sum is 3 →hybridization−sp2 If the sum is 4 →hybridization−sp3 If the sum is 5 →hybrid...
Transcribed image text: below are the four molecular orbitals of butadiene. Which two orbitals are occupied with electrons ? g6 & 88 & gf & g88 8888 D c B A A. Cand D B. A and c C. A and D D. Bandc Select the product of this reaction: 1 HCN NaCl 2.

The National Cancer Institute's Natural Products Branch at the Frederick National Laboratory for Cancer Research is the largest program to collect materials worldwide from marine, plant, and microbial sources so they may be studied for possible medical uses.
Oct 06, 2020 · an infamous small molecule known as hydrogen cyanide is shown draw a molecular orbital diagram. then draw MOs of the HCN molecule in the middle. indicate which MO is LUMO AND HOMO The molecular orbital diagram of NO shown in Figure 10.47 also applies to OF .
CH 10 Bonding & Molecular Structure: Orbital Hybridization & Molecular Orbitals. a) MO theory predicts that electrons are delocalized over the molecule. b) VB theory predicts that oxygen is paramagnetic, MO theory does not. c) VB theory describes a molecular bond as the overlap between two atomic orbitals.
Energy level diagram of HCN (a) and the HCN-water complex (b). Molecular orbitals (MOs) marked in red and black are included in the active spaces for RIXS ...
Microcentrifuge tubes in a rack. Some of them are DNA samples while the remainder of them are primers to be used in polymerase chain reaction, or PCR, a laboratory technique used to make multiple copies of a segment of DNA.
The sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to one of the 2p atomic orbitals. The combination of these atomic orbitals creates three new hybrid orbitals equal in energy-level. Which of the following will show sp2 hybridization? Sulphur in SO2, is sp2 ...
Download scientific diagram | HOMO and LUMO orbitals of HCN molecule on the Mg and Ca-doped BeONTs from publication: Boosting BeONT Reactivity with HCN by Calcium and Magnesium Doping: A DFT ...
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the chemical formula HCN. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F).HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals.
by DC Pan · 1967 · Cited by 50 — A linear molecule with bond distances correct to 1% of the microwave spectroscopy values is obtained. Walsh's orbital-energy diagrams are found to be almost ...
Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN– (Cyanide ion), NO+ (Nitrosonium ion).
Nov 08, · RE: a thank you to entice the C-N molecular orbital diagram for the sigma bond in HCN? Label each and every molecular orbital with its call (sigma, pi) and place the obtainable electrons interior the proper atomic orbitals and molecular wiringall.com: Resolved. Steps in predicting the hybrid orbitals used by an atom in bonding: 1.

A technician using a microtome at the Advanced Technology Research Facility (ATRF), Frederick National Laboratory for Cancer Research, National Cancer Institute. A microtome is an instrument that cuts extremely thin sections of material for examination under a microscope.
Mar 25, 2021 · Generally, molecules with linear molecular geometry have sp hybridization as the central atom forms bonds with two atoms only. Number of sigma bonds in the molecule. Another way to find out the hybridization of the molecule is to look at the number of sigma bonds formed. In HCN, there are two sigma bonds, C-H and C-N. The number of sigma bonds is equal to the number of hybrid orbitals formed.


















0 Response to "37 hcn molecular orbital diagram"
Post a Comment