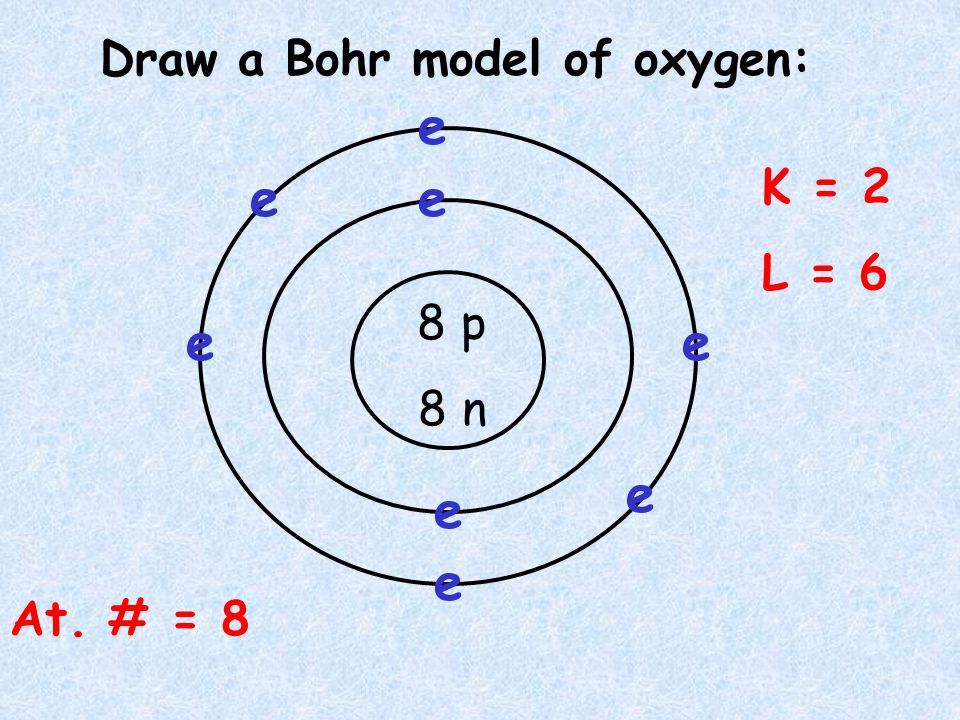
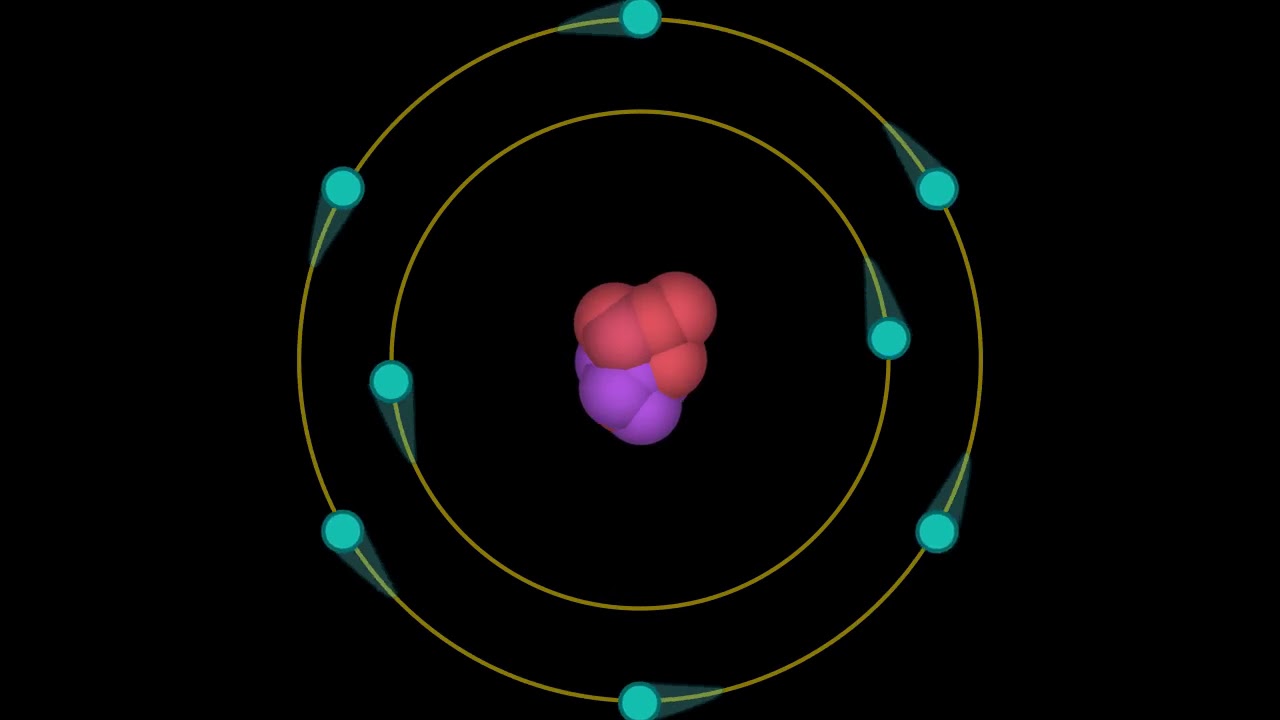
39 bohr diagram of oxygen
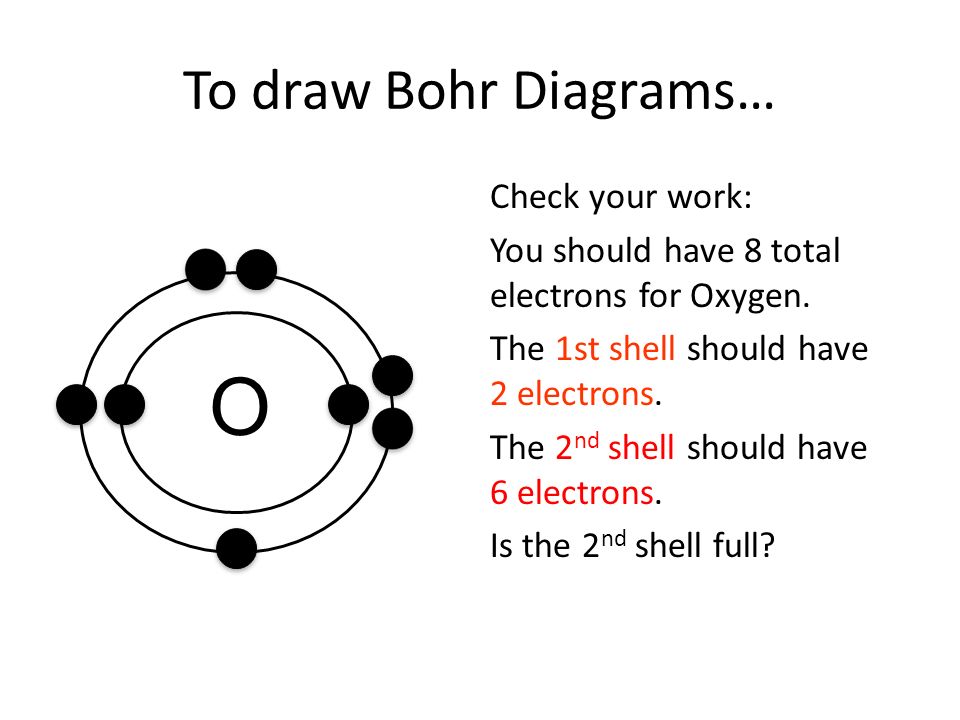
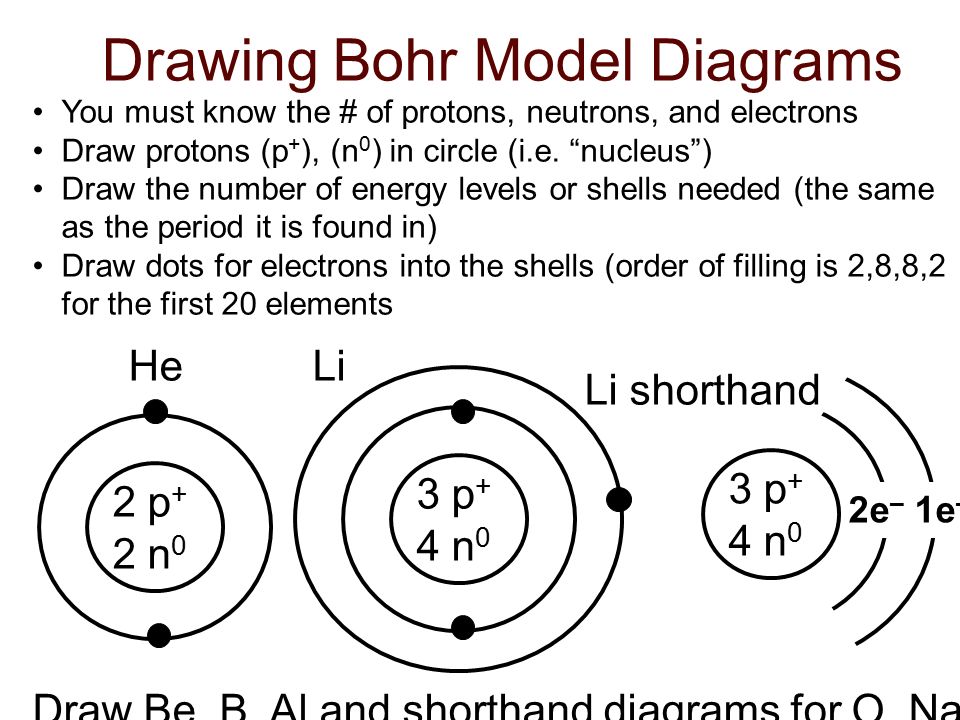
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Feb 15, 2016 - How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
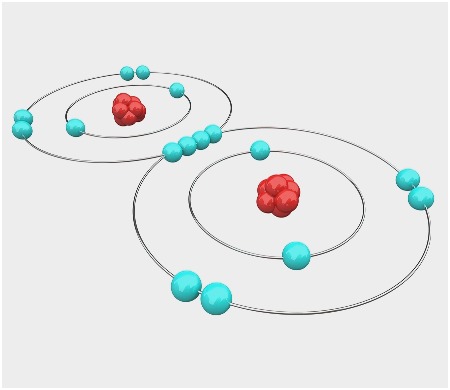
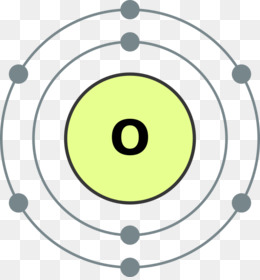
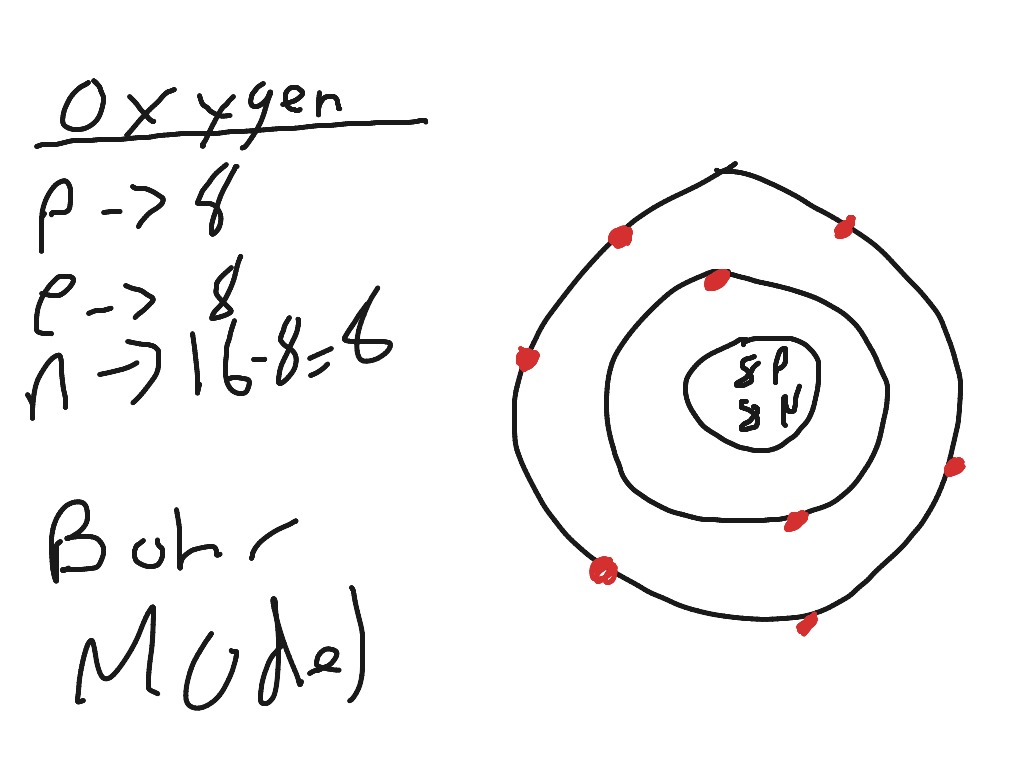
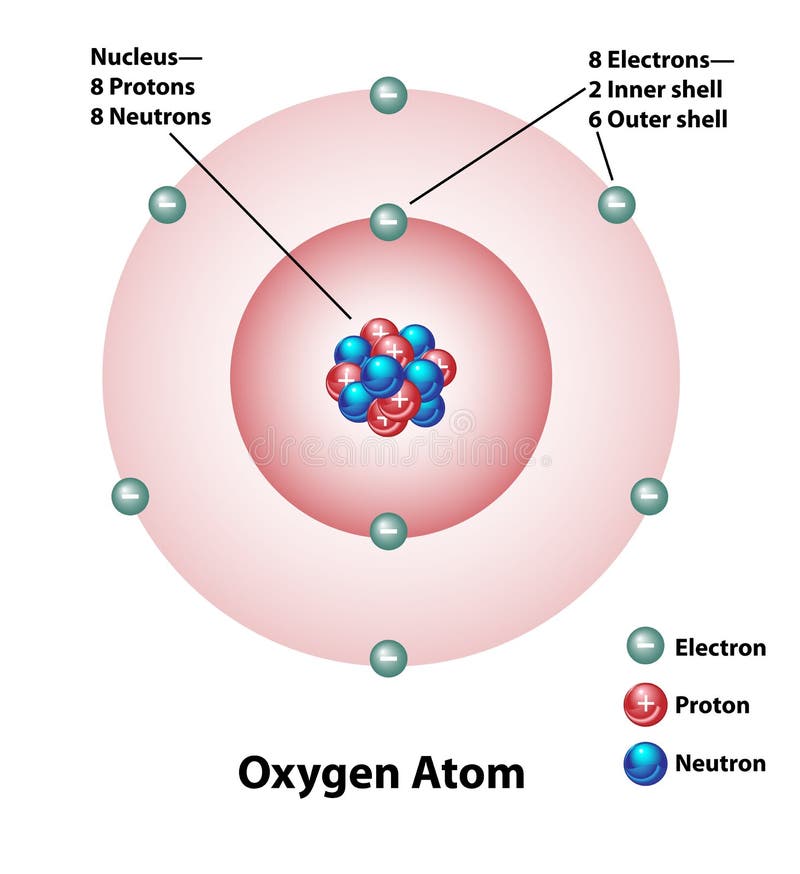
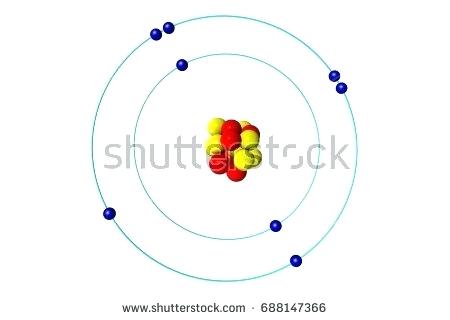
1 answerThe Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels....

Bohr diagram of oxygen
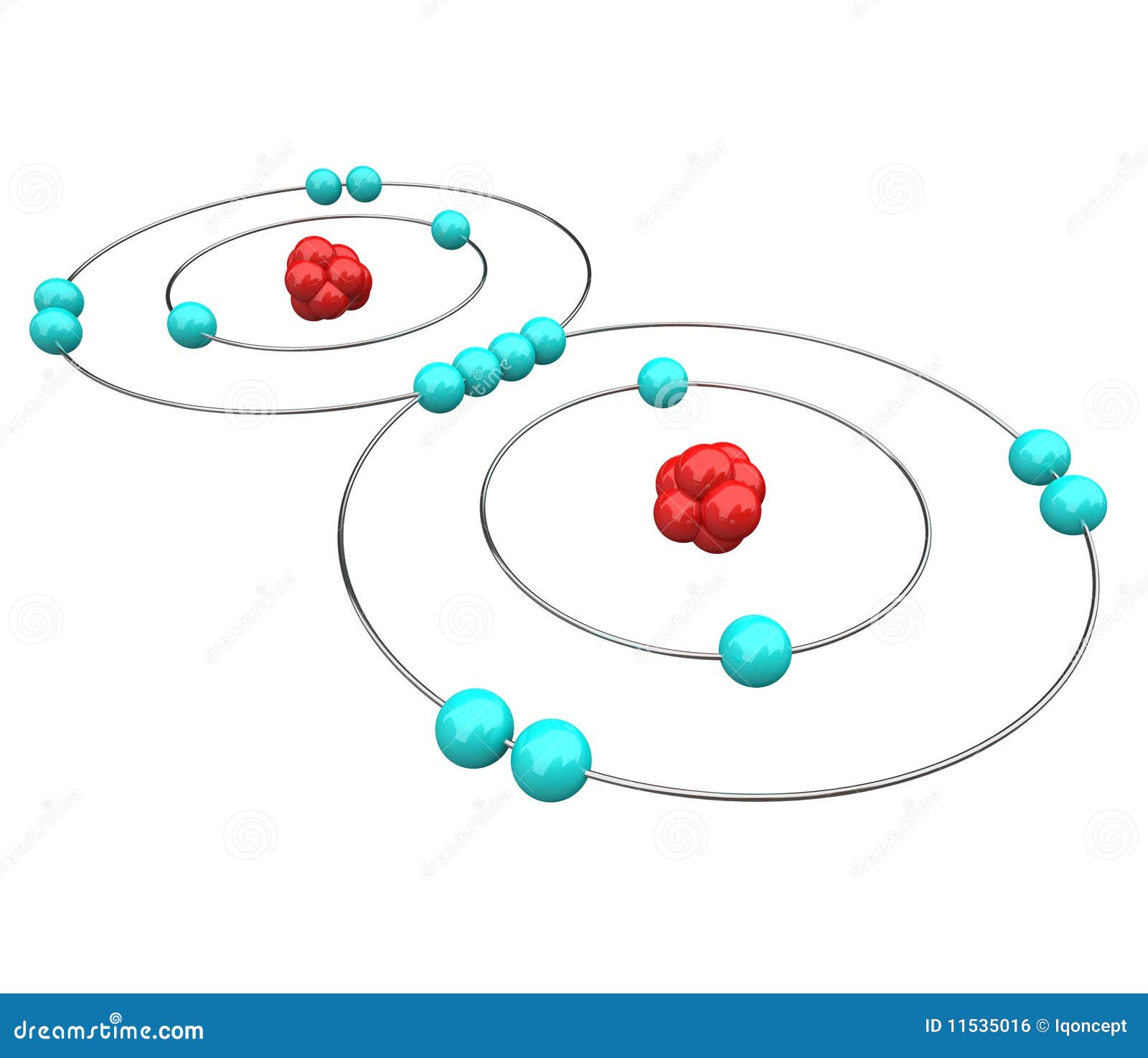
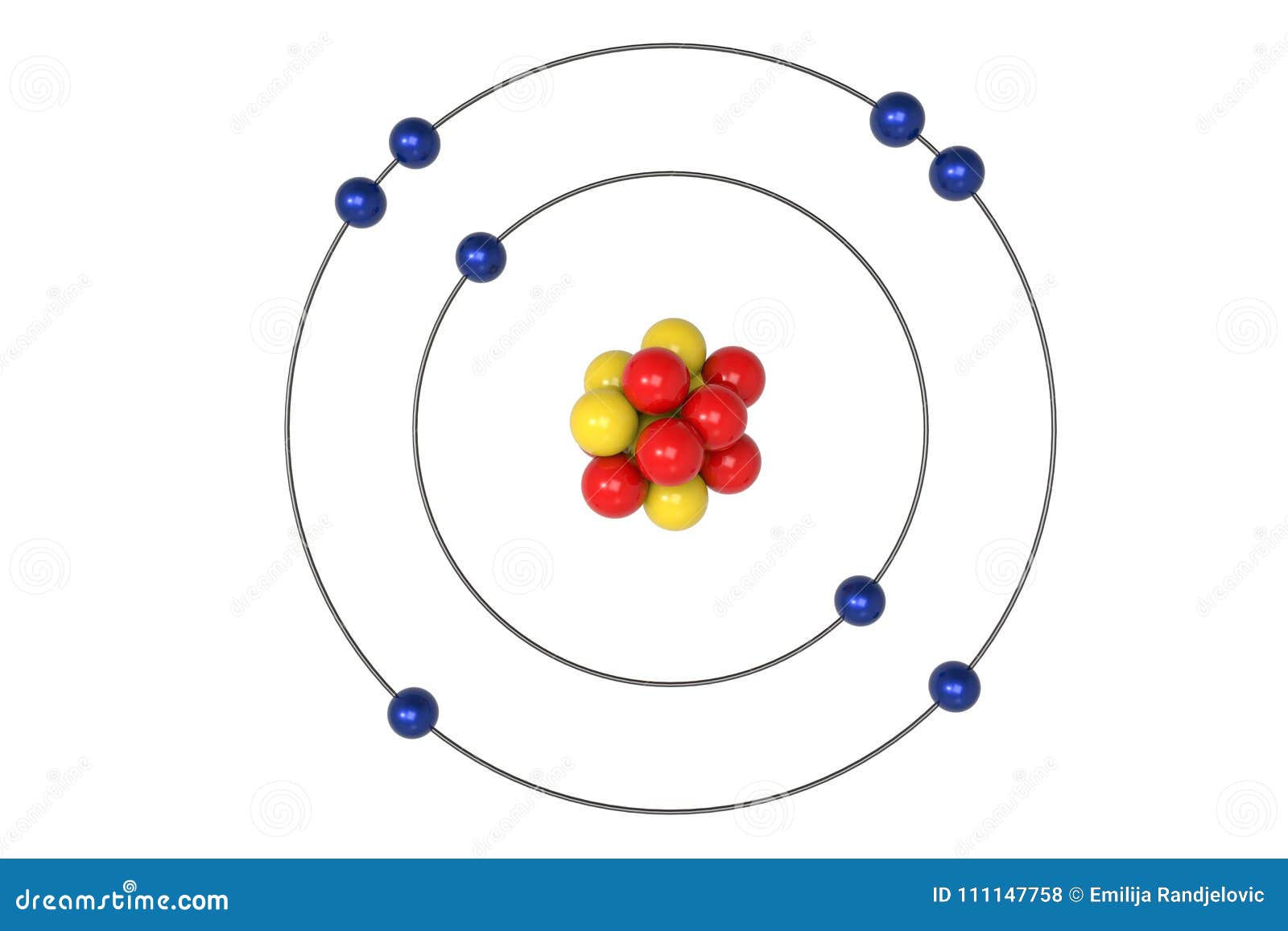
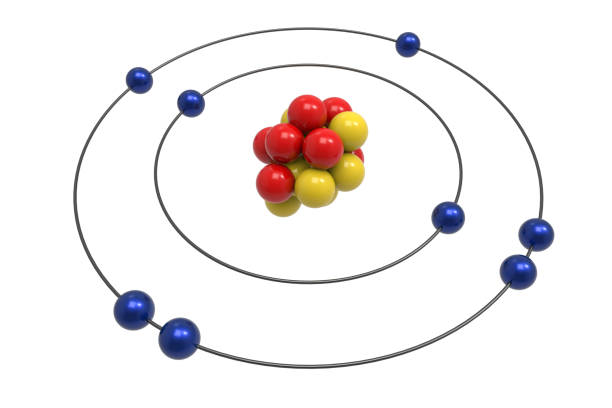
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants. Oxygen - Atomic Diagram Carbon Atom Bohr model with proton, neutron and electron. 3d illustration Bohr model of Nitrogen Atom with proton, neutron and electron.
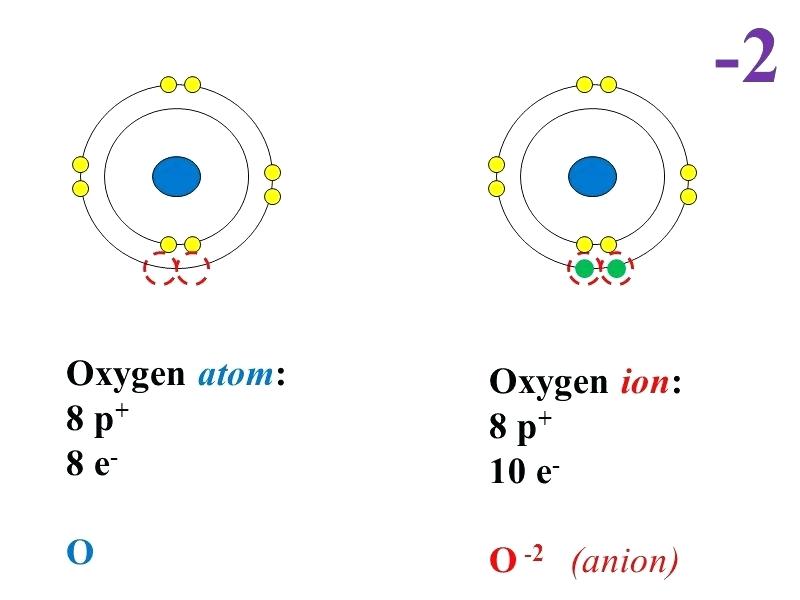
Bohr diagram of oxygen. Technology merges into biology and science discovers the last secrets of existence. Nature is no longer considered as the exclusive owner and producer of the human body. We witness the dawn of a new era. It is the search for a second nature interpreting the body as a construction of men made parts. The research of Brach resulted in a book about ... Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8, 1: 12: Bohr model of Magnesium (Mg) 2, 8, 2: 13: Bohr model of ... Lewis Dot Diagrams… Gilbert Lewis used a different model than Bohr, and he only showed the valence e- in it. His model is called the . Lewis dot structure . He put dots around the symbols so that we can . see. just the . valence electrons . for the elements (so we can easily see which e- are going to react) This is the answer to the Stop and Jot from Lesson 1.4 for the Bohr Electron Configuration Drawing of the Oxygen Ion
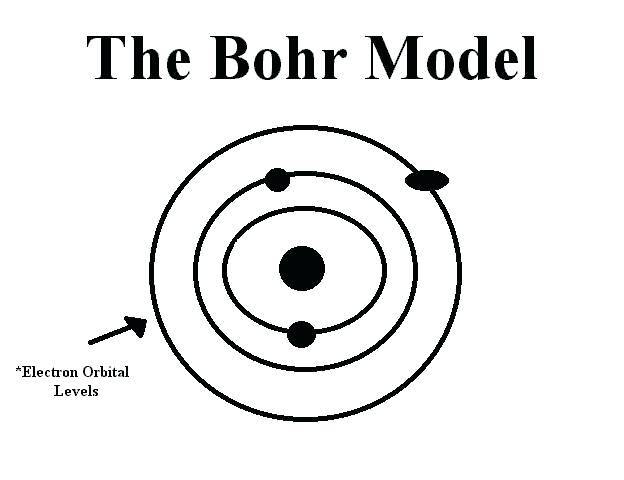
Draw the Bohr model for Oxygen; Draw the Bohr model for Lithium; P = N = P = N = Bohr Diagrams. You should know how to draw a Bohr Diagram for the first 20 elements. On page 12 make the following table and choose an element from each of the first three rows of the periodic table to complete the table ... The Bohr effect is a phenomenon first described in 1904 by the Danish physiologist Christian Bohr. Hemoglobin's oxygen binding affinity (see oxygen-haemoglobin dissociation curve) is inversely related both to acidity and to the concentration of carbon dioxide. That is, the Bohr effect refers to the shift in the oxygen dissociation curve caused by changes in the concentration of carbon ... how do you draw a bohr model of oxygen answers for neutral oxygen the bohr model would have a circle with 8 inside because oxygen is atomic number eight and has eight protons two electrons in the first orbit and six in the second orbit to make eight Oxygen Bohr Diagram - Cross Linking With O Raffinose Lowers Oxygen Affinity And Stabilizes A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole. This is because protons and neutrons ... What is the Bohr diagram for oxygen? The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the. What is the Bohr diagram for oxygen? The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the. What is the Bohr diagram for oxygen? - FindAnyAnswer.com. Jun 23, 2020 · The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels.
Jun 23, 2020 · What is the Bohr diagram for oxygen? The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels. Accordingly, what is a Bohr diagram? A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913.
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levels. Click to see full answer Keeping this in view, what is a Bohr diagram? A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913.
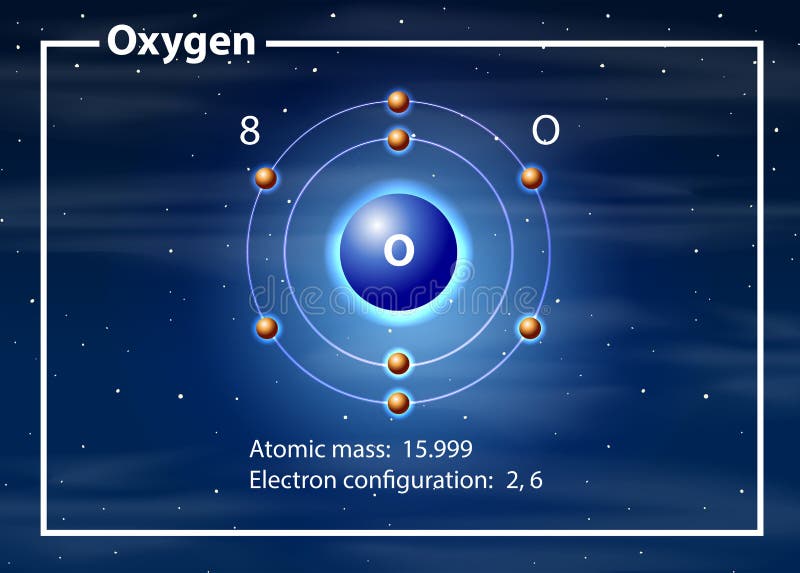
The Bohr Model of Oxygen (O) has a nucleus that contains 8 neutrons and 8 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr...
Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point:-218.4 °C (54.750008 K, -361.12 °F) Boiling Point:-183.0 °C (90.15 K, -297.4 °F) Number of Protons/Electrons: 8 Number of Neutrons: 8 Classification: Non-metal Crystal Structure: Cubic Density @ 293 K: 1.429 g/cm 3 Color: colorless Atomic Structure
Bohr Diagram Oxygen ... 960x720 0 0. Like JPG. Bohr Diagram For Oxy... 1466x1800 0 0. Like JPG. Bohr Model Oxygen Sc... 1024x768 0 0. Like JPG. Bohr Model Descripti... 600x400 0 0. Like JPG. Diagram Example Of A... 474x613 0 0. Like JPG. Diagram Of The Eye Q... 800x600 0 0. Like JPG. Diagram Of The Heart... 638x479 0 0. Like JPG. Do And Answer ...
The Bohr Model of Oxygen(O) has a nucleus that contains 8 neutrons and 8 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell.Total valence electrons in Oxygen: 6Total electron shells: 2Electrons in the Second shell(L): 6Number of electrons: 8Steps to draw the Bohr Model... · Find Valence electron of...
Bohr Diagrams • A Bohr diagram is a diagram that shows how many _____ are in each shell surrounding the nucleus. • Named in honour of _____, a Danish physicist who developed several models for showing the arrangement of electrons in atoms. • There are three main background questions to explore before we start drawing Bohr diagrams.
We collected 40+ Bohr Model Drawing Oxygen paintings in our online museum of paintings - PaintingValley.com. ADVERTISEMENT. LIMITED OFFER: Get 10 free Shutterstock images - PICK10FREE. oxygen. model. bohr. diagram. atomic. rutherford.
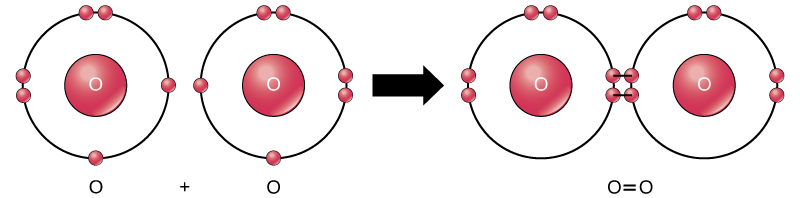
•When elements form compounds, changes occur in the arrangement of electrons in the outer orbit. • Electrons are gained or lost so that element can have a stable electron arrangement of the closest noble gas. • Atoms prefer a completely filled outer shell with electrons • In order for a compound to be stable, it must have a completely filled ...
How to draw the Bohr-Rutherford Diagram for Oxygen. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
The electron configuration of oxygen in Hund’s principle is 1s 2 2s 2 2p x2 2p y1 2p z1. The electron configuration of oxygen in excited state is O* (8) = 1s 2 2s 2 2p x2 2p y1 2p z1. The last orbital of oxygen is ‘p’. And unpaired electrons exist in its last p-orbital. So, the oxygen atom supports the Hund principle.
Rutherford-Bohr model. The atomic number of oxygen is 8 therefore this element is represented by 8 protons (positive charges) in the nucleus and 8 electrons (negative charges) that are distributed as follows: - 2 electrons on the first shell - 6 electrons on the second shell. Figure 1 RUTHERFORD-BOHR DIAGRAM OF AN OXYGEN ATOM
Oxygen - Atomic Diagram Carbon Atom Bohr model with proton, neutron and electron. 3d illustration Bohr model of Nitrogen Atom with proton, neutron and electron.
The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
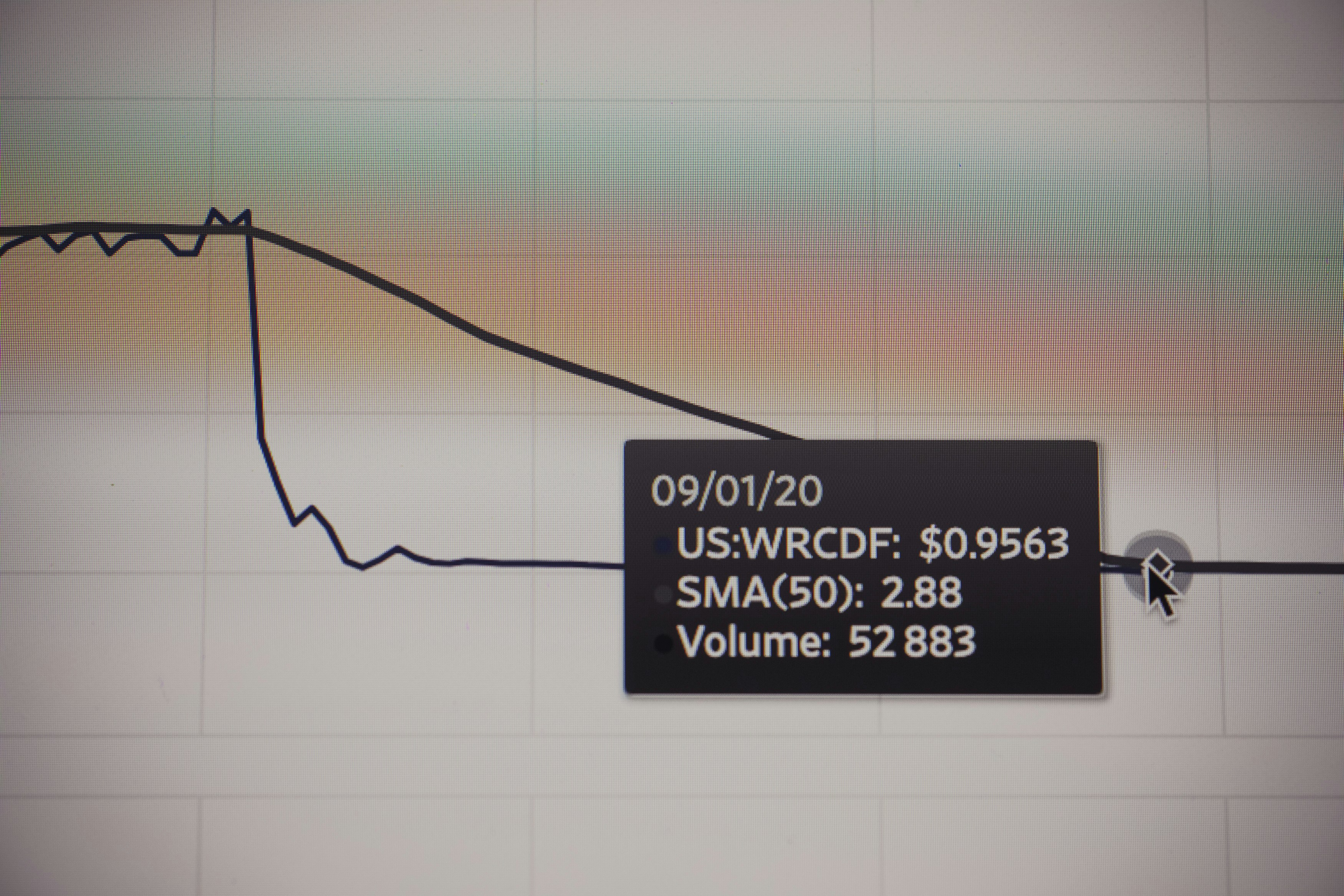
Finance investment stock market chart. Made with analog vintage lens, Leica APO Macro Elmarit-R 2.8 100mm (Year: 1993)












0 Response to "39 bohr diagram of oxygen"
Post a Comment