41 label the following reaction energy diagram for a catalyzed and an uncatalyzed process
Sulfur (in British English: sulphur) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic.Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S 8.Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most common element by mass in the universe and the fifth ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Arx > 0 Transition Stato Reactants Entwu no catalyst Entwd) with catalyst Products Potential energy Unentalyzed Entravno catalyst Reaction Intermediate Catalyzed AH 0 Earov) with catalyst Reaction progress
Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea (fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea (rev) no catalyst Ea ...
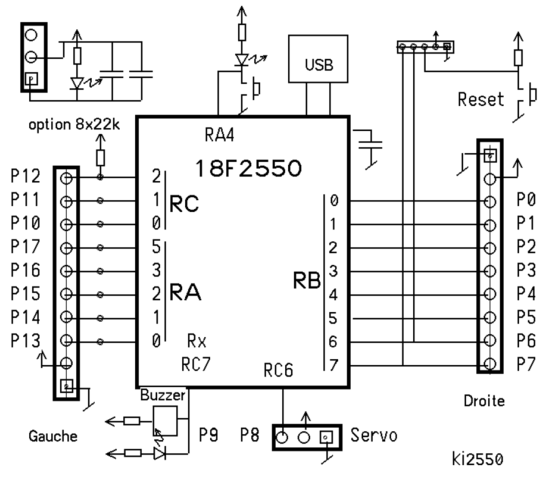
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process
Best Answer. This is the best answer based on feedback and ratings. 89% (38 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Previous question Next question. Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ... Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Reactants State Products Catalyzed Uncatalyzed | | ΔHnn < 0 Ea(lwd) no catalyst Ea(rev) no catalyst Fattwo) with Ea(rev) With catalyst catalyst Reaction AHrxn 0 Intermediate Reaction progress
Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Learn this topic by watching Energy Diagram Concept Videos. Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Reactants State Products Catalyzed Uncatalyzed | | ΔHnn < 0 Ea(lwd) no catalyst Ea(rev) no catalyst Fattwo) with Ea(rev) With catalyst catalyst Reaction AHrxn 0 Intermediate Reaction progress Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ... Best Answer. This is the best answer based on feedback and ratings. 89% (38 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Previous question Next question.

0 Response to "41 label the following reaction energy diagram for a catalyzed and an uncatalyzed process"
Post a Comment