41 mo diagram for cn-
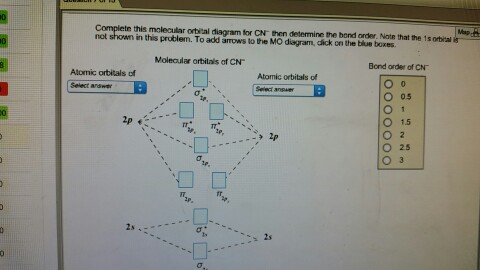
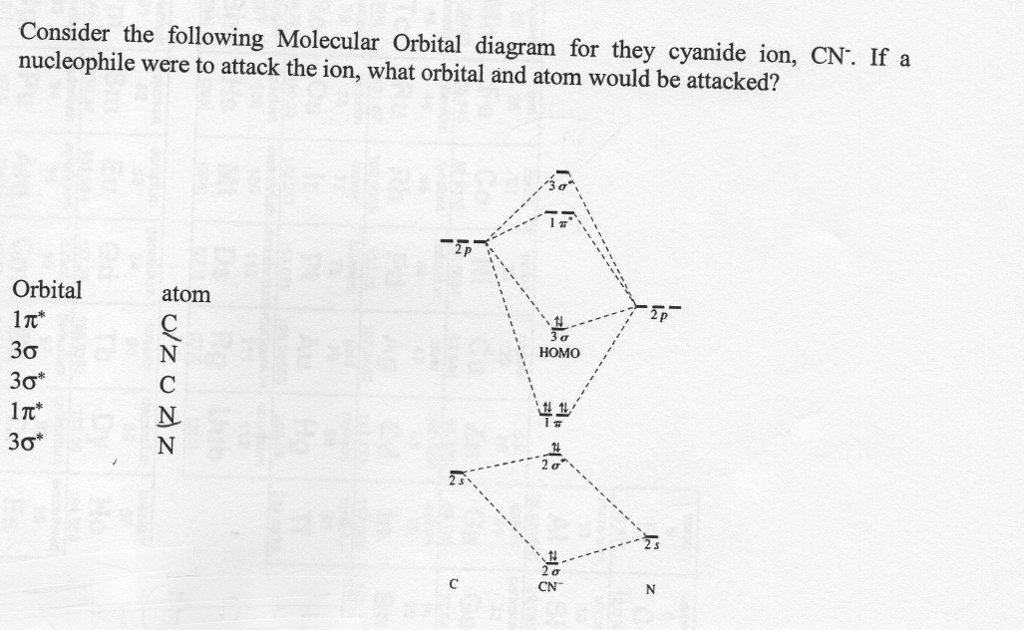
MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in carbon is six. Science. Chemistry. Chemistry questions and answers. Assignment Score: 36.9% Resources Hint Check Answer < Question 7 of 16 > A partial MO diagram is shown for CN. Place the 2p atomic orbital for carbon in the correct location Which orbital in the diagram is the HOMO?
MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
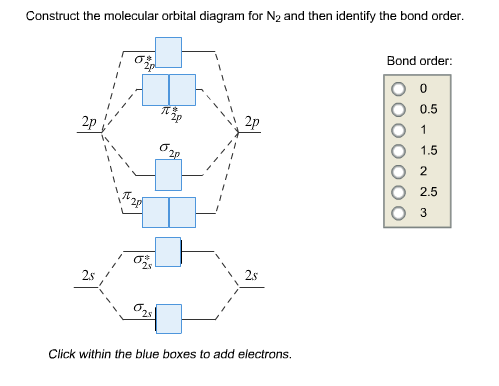
Mo diagram for cn-
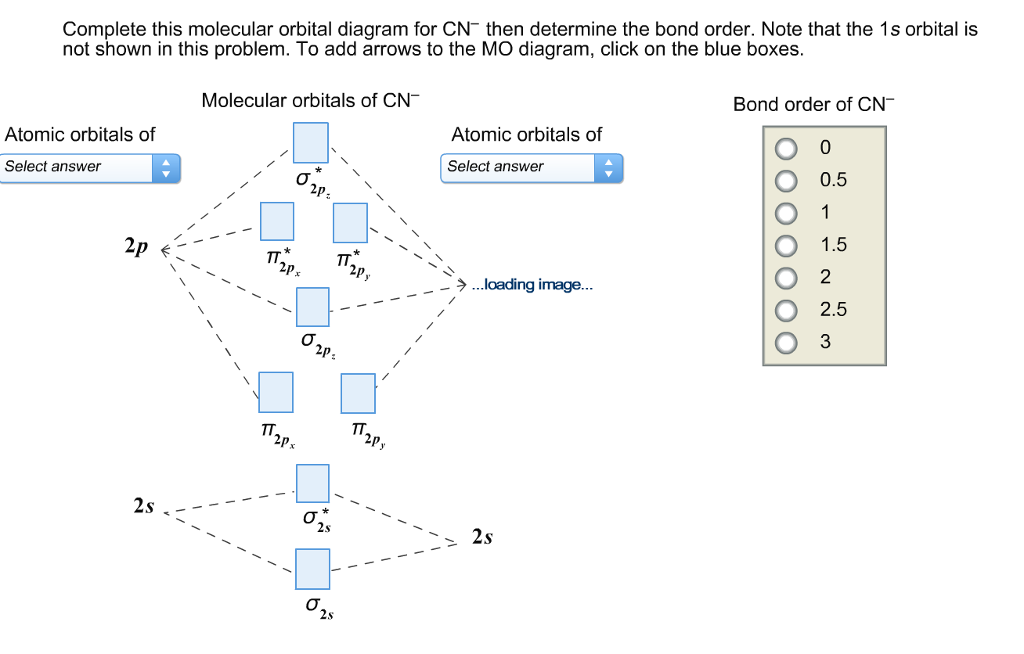
Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B. O. = 8 − 2 2 = 3 Here's a guide on how to construct MO diagrams, in case you need help. 57.6K views View upvotes View 1 share Kakali Ghosh , High School Teacher at Schools CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN). The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ...
Mo diagram for cn-. MO Diagram of CN-< Theoretical chemistry research group focusing on development of methods, and calculations in the areas of ionic liquids, photochemistry and catalysis step 9 Molecular Orbital Mixing More detail was added to this answer in response to input and questions from students in the class of 2008. Molecular Orbital Diagram of CO. By. All About Chemistry - July 2, 2020. 1. 237. Molecular Orbital Diagram of CO. TAGS; Molecular Orbital Diagram; Previous article Wohl-Ziegler Bromination. Next article Molecular Orbital Diagram of NO. All About Chemistry. https://allaboutchemistry.net. Hello Reader! Thanking for reading this post, If you find ... This video is about MO Diagram #3 - CN- $\begingroup$ No, $\ce{CN-}$ is the case in point. From an MO picture, there is no non-bonding "lone pair." There's nothing to donate. Coordination between a metal and $\ce{CN-}$ in MO-world and Lewis structure world look very different. The end-result of MO and Valence Bond may be similar, but the interpretations are different. $\endgroup$
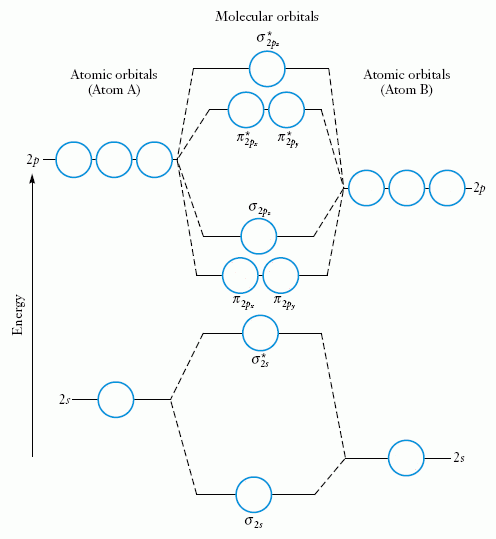
Stable electronic configurations: MO Energy Level Diagrams Reviewed . Electron count preference . Electron count and Oxidation States Stable electronic configurations: MO Energy Level Diagrams Reviewed Electron count preference Electron count and Oxidation States Ligands • Carbon Monoxide • Phosphines Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion). Figure \ (\PageIndex {2}\): Molecular Orbital Energy-Level Diagram for \ (\ PageIndex {12}\) to describe the bonding in the cyanide ion (CN −). In the traditional Lewis picture there are two lone pairs on the cyanide ion. Yang Chen-Ning or Chen-Ning Yang (Chinese: 杨振宁; pinyin: Yáng Zhènníng; born 1 October 1922), also known as C. N. Yang or by the English name Frank Yang, is a Chinese theoretical physicist who made significant contributions to statistical mechanics, integrable systems, gauge theory, and both particle physics and condensed matter physics.
The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron. You can either draw the `O_2` diagram and remove `1` electron, or just draw the `O_2^+` diagram. The diagram will end up as such: Notice the effect that this has on the overall bonds. The novel high-entropy alloys (HEAs) with compositions of (Ti 1/3 Zr 1/3 Hf 1/3) 15 (Nb 1/2 Ta 1/2) x (x = 3, 5) for biomedical applications have been designed and synthesized, using the cluster formula approach. The present HEAs have a single body-centered-cubic (BCC) solid-solution structure and possess prominent mechanical properties of high compressive yield strength … Interactive Exercise: Drawing the MO diagram of CN-Right, you have been asked to draw the MO diagram for CN-, a heteronuclear diatomic. Don't panic! Take it one step at a time and you will have a complete MO diagram before you know it. This is meant to be an interactive exercise, so arrange for some pieces of blank paper, a pencil, a pen and an ... Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2 + CH 2, SiH 2, BH 2 6 Bent-, NH2 + BH 2, AlH 2, CH 2 5 Bent BeH 2, BH 2+ 4 Linear LiH 2, BeH 2+ 3 Linear LiH 2+ 2 Bent No. of Shape valence electrons Molecular species Known shape of some AH 2 molecules Recall: a molecule adopts the structure that best stabilises the HOMO.
Module Two Chem 101 Problems. TestNew stuff! Carbon dioxide is a _____ compound composed two types of _____ atoms. Carbon dioxide is a molecular compound composed of two oxygen atoms with covalent double bonds to a central carbon atom. Both C and O are to the right of the "staircase" on the periodic table, as nonmetals.
How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s
In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc. It is released as a decay product of many plants and it is one of the most poisonous chemicals in chemistry. Some bacteria, fungi, …
Q. Draw Lewis structures and MO diagrams for CN+, CN, and CN-. According to the Lewis model, which species is most stable? Q. Use molecular orbital theory to complete this table. Q. What is the ground-state electron configuration of O2^-? 1. (δ2s)^2(δ2s*)^2(π2p)^3(π2p*)^1 2.
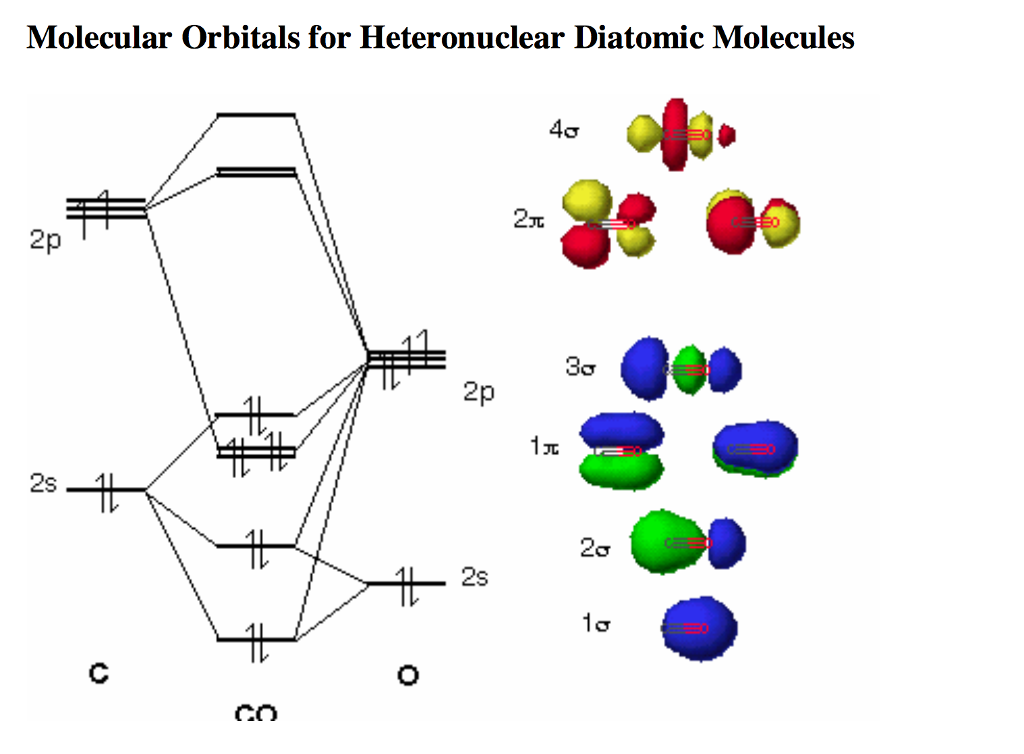
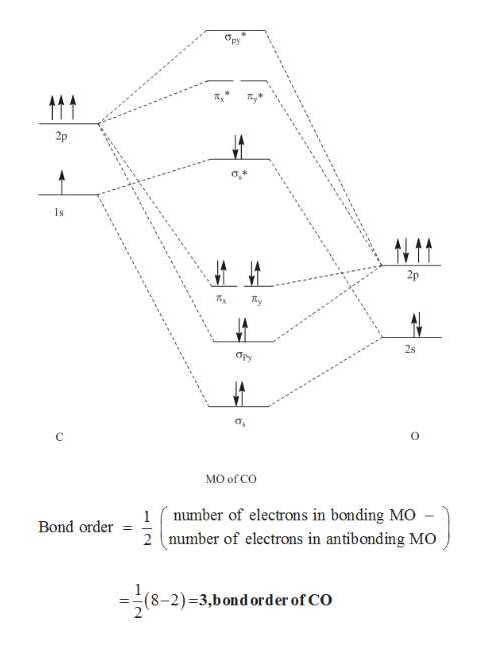
The Molecule. CO is a very stable 10-valence-electron molecule, isoelectronic with [CN] - and with N 2, which has a slightly lower bond dissociation energy than CO. The formal bond order of CO is 3, from about one σ- bond and two π- bonds. Its most important property is burning in air to give CO 2 , in the combustion of fossil fuels.
Tania Havenga. University of South Africa. Here is the MO for CN-, just take away a single electron from the MO since CN is neutral. Vijayta Gupta is right, the N atom is lower in energy. http ...
Chemistry (1st Edition) Edit edition Solutions for Chapter 7 Problem 66QP: Draw the molecular orbital diagram for the cyanide ion (CN−). (Assume the ordering of moleculer orbitals to be like that in N2.) Write the electron configuration of the cyanide ion (CN−). ….
Explain the MO diagram for NO molecule. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 23, 2020 by Taashi (15.8k points) selected Dec 24, 2020 by Aashi01 . Best answer. 1. Electronic configuration of N atom is 1s 2 2s 2 2p 3 . 2. ...
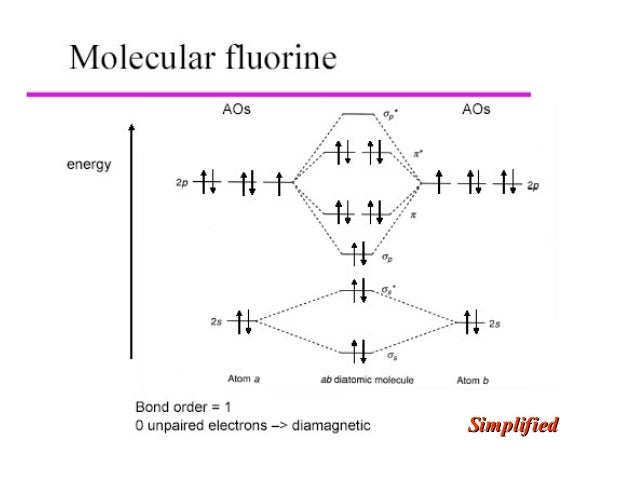
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Molecular orbital Diagram Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory heteronuclear ...
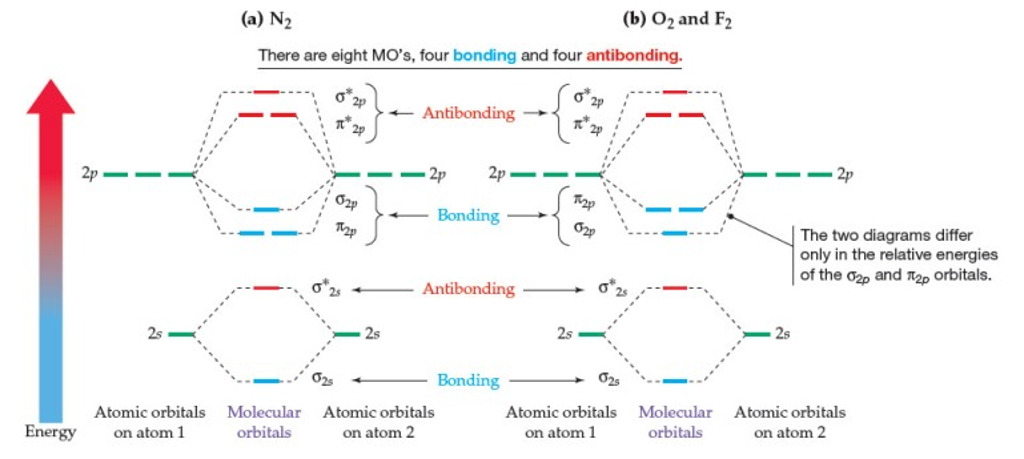
Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. Figure 11. This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective nuclear charge increases and atomic ...
06.02.2016 · CCNA 4 Final Exam Answers 2019 2020 version 5.1 v6.0 2017 - 2018 100% Full, CCNA v5.0.2 v5.0.3. CCNA 4 Connecting Networks. New Questions updated latest pdf
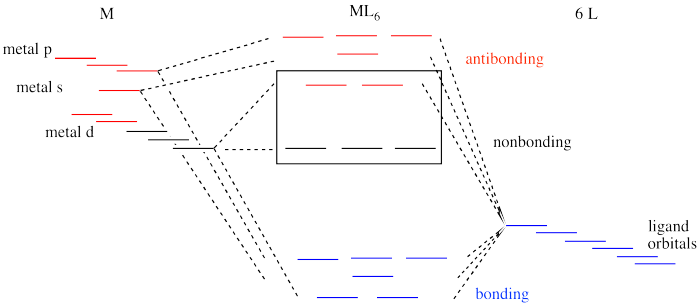
Example: Constructing a MO diagram for Chromium Hexacarbonyl, Cr(CO) 6 Cr ππππ-bonding AOs T2g:(3dxy,3dxz,3dyz) T1u:(4px,4py,4pz) • T2g previouslyconsiderednon-bondingin σ-bondingscheme • T1ucombineswith T1u SALCin in σ-bondingscheme • T1g, T2u π-SALCs are non-bonding Cr non-bonding AOs T2g: (3 dxy, 3dxz, 3dyz) Cr σσσσ-bonding ...

Image from page 510 of "Mechanics of engineering. Comprising statics and dynamics of solids: and the mechanics of the materials of constructions, or strength and elasticity of beams, columns, arches, shafts, etc" (1888)
The molecular orbital configuration of C N + is K K σ (2 s) 2, σ ∗ (2 s) 2, π (2 p x ) 2, π (2 p y ) 2. Bond order is 2 . All the electrons are paired and ion is diamagnetic.
The MO diagram for "NO" is as follows (Miessler et al., Answer Key): (The original was this; I added the orbital depictions and symmetry labels. For further discussion on the orbital energy ordering being "N"_2-like, see here and comments.) Quick overview of what the labels correspond to what MOs: 1a_1 is the sigma_(2s) bonding MO. 2a_1 is the sigma_(2s)^"*" antibonding MO. 1b_1 is the pi_(2p ...
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore B. O. = 8 − 2 2 = 3 Here's a guide on how to construct MO diagrams, in case you need help. 57.6K views View upvotes View 1 share Kakali Ghosh , High School Teacher at Schools












![DFT energy level diagram for [Mo 5 NbI 8 (CN) 6 ] 3 ...](https://www.researchgate.net/profile/Sofya_Artemkina/publication/257859841/figure/download/fig4/AS:297638919458823@1447974056584/DFT-energy-level-diagram-for-Mo-5-NbI-8-CN-6-3-schematic.png)


![The structure of: (a) [Mo(CN) 6 (H 2 O)] 2) ; (b) [Mo(CN ...](https://www.researchgate.net/profile/Tomasz_Stawski/publication/239116740/figure/fig1/AS:655139761311763@1533208899773/The-structure-of-WbpyCN-6-ion-50-probability-ellipsoids_Q640.jpg)
0 Response to "41 mo diagram for cn-"
Post a Comment