37 lewis dot diagram for barium
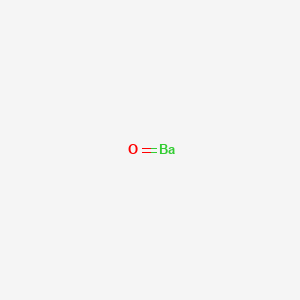
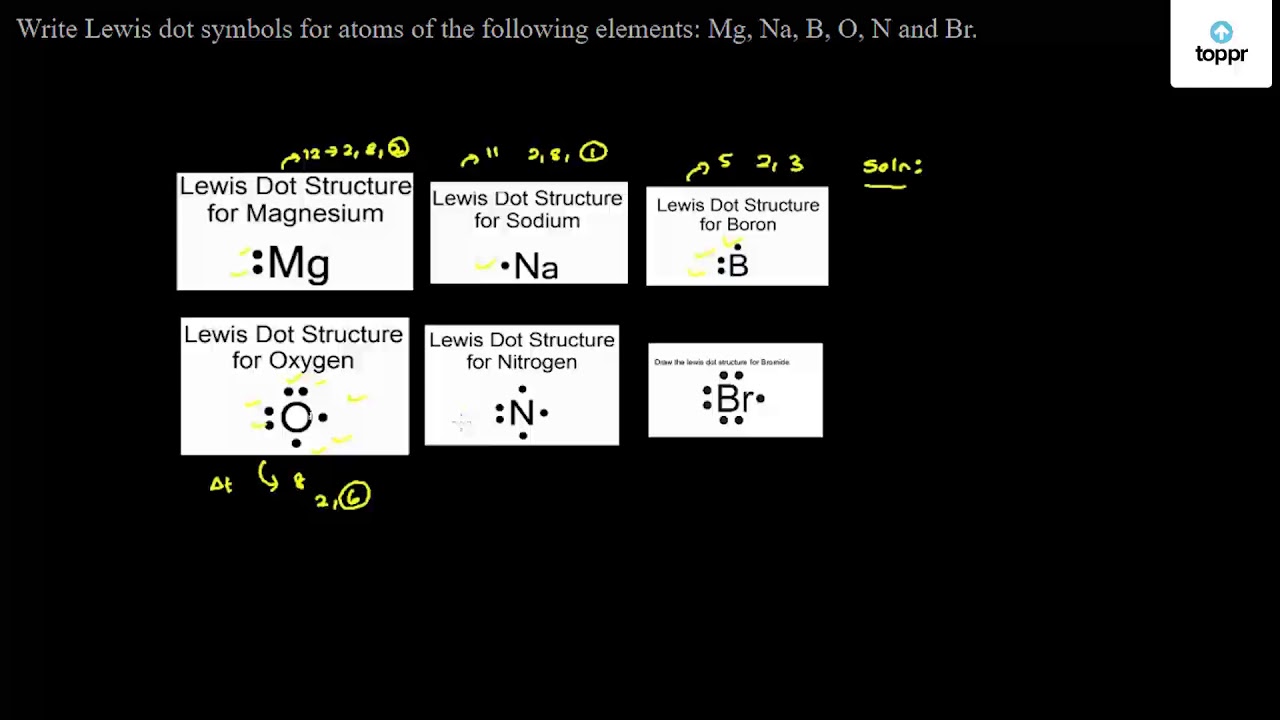
Barium is in Group 2 .as it is in Group 2 it will contain 2 valence electrons. When you sketch the Lewis arrangement for Barium you'll place two "dots" or valance electrons approximately the element symbol (Ba) 396 views Sponsored by BHMD Deep Wrinkle Plastic surgeon shares one weird way to fill in wrinkles at home. Draw an electron-dot structure for an atom of each element. A. carbon B. arsenic C. polonium D. potassium E. barium. Explanation. Verified. Step 1.1 answer · Top answer: $\textbf{a)}$ Carbon : $\mathrm{\left [ He ight ]2s^22p^2}$ - 4 valence electrons. $\textbf{b)}$ Arsenic : $\mathrm{\left [ Ar ight ]4s^23d^{10}4p^{3}}$ ...
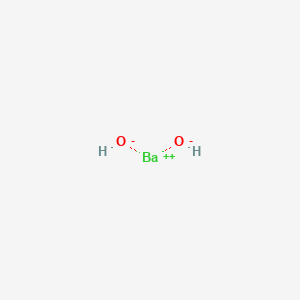
I searched for $\ce{BaF2}$ Lewis structure, and found out that the Barium atom loses its 2 valence electrons to form $\ce{F- Ba^2+F-}$. Before seeing it I thought each of $\ce{Ba}$'s valence electr...
Lewis dot diagram for barium
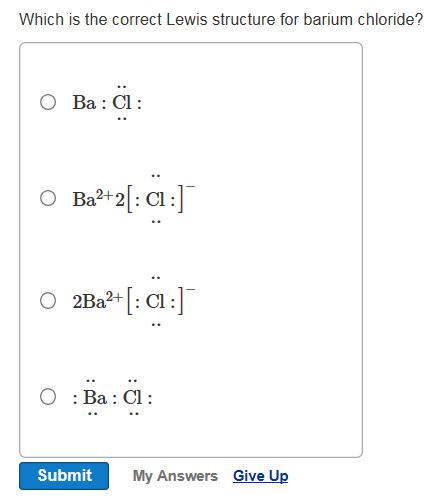
A step-by-step explanation of how to draw the BaCl2 Lewis Dot Structure.For BaCl2 we have an ionic compound and we need to take that into account when we dra... Orbital Diagram For Barium The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium. Barium is an alkaline earth metal. This means that it is a group 2 element. Barium's atomic number is 56; this means that it has 56 protons in its nucleus and also. Lewis dot structure of the potassiumis to be drawn. Concept introduction: Lewis structure is a representation of a chemical formula of substance with valance electrons of atoms. The Lewis structures are also called electron dot structures. In the Lewis structure, electrons are denoted by dots.
Lewis dot diagram for barium. 9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on. Transcribed Image Text. Write the Lewis dot Symbol for the representation of the formation of barium hydride. 2. Which of the following bonds is covalent, which is polar covalent and which is ionic? (a) the bond in CsCl, (b) the bond in H2S, (c) the NN nond in H2NNH2. 3. Write the Lewis structure for formic acid (HCOOH) and nitrite ion (NO2 ). 4. The lewis structure is F:Ba:F just draw the remaining electrons around the fluorine atoms (there is a total of 16 electrons in this compound). It is also non-polar (fluorine is most electronegative element in the periodic table and barium is not very electronegative but due to symmetry, the molecule is not polar). 1. a) Draw the Lewis dot structure for an atom of carbon and an atom of bromine. b) Using the NEED, HAVE, SHARE method, determine the number of valence electrons that would be shared between one carbon atom and four bromine atoms. c) Draw the Lewis dot structure for the compound CBr 4. 2. a) Draw the Lewis dot structure for an atom of iodine.
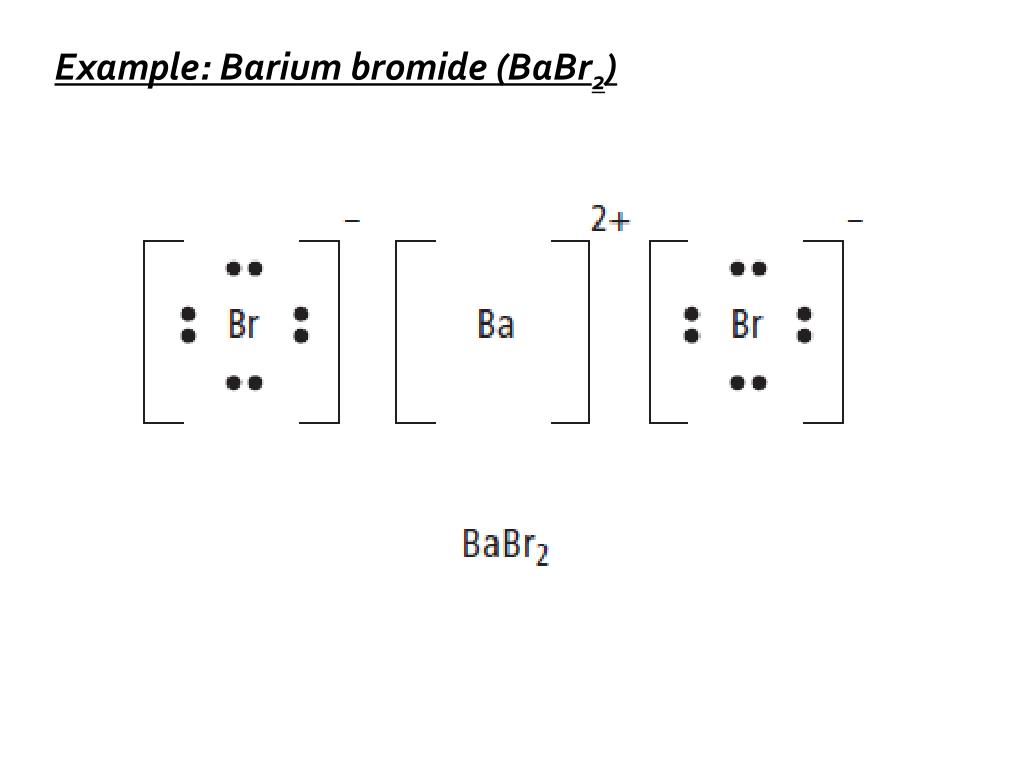
Barium And Oxygen Lewis Dot Diagram, Lewis Dot Structure for Barium (Ba) YouTube, Lewis Dot Diagram For Cl Atkinsjewelry, What is the purpose of an electron dot diagram? Quora, into the Rafflesian Chemist's mind: February 2011 239 People Used More Info ›› Visit site ... Answer to: Draw the transfer of electrons within barium sulfide using a Lewis dot structure and write the chemical formula. By signing up, you'll...1 answer · Top answer: Barium sulphide can be considered an ionic compound. Barium has two valence electrons in its valence shell. Barium transfers its two valence electrons... Lewis Structure the ionic compound of Barium fluoride 4: Add more atoms if needed If the transfer from one atom to another doesn't result in full outer shells, add more atoms Ba F Barium has 2 electron Fluorine has 7 electrons Example: Draw the Lewis Structure the ionic compound of Barium fluoride The fluorine is full, but the Barium isn't! Lewis Structure Practice Worksheet 1 Answers Workssheet List. Barium phosphide (ba3p2) tribarium diphosphide. ifsa porno so, as far as lewis dot structures are concerned, nitride will have four lone pair of iodide, lewis dot structure for barium phosphide, barium hydride lewis dot . unit 3 compounds victor valley college.
The absorption of specific barium salt anions /was examined/ in male Sprague-Dawley rats administered radiolabelled barium chloride, sulfate, or carbonate to fasted (24 hr) and non-fasted rats /strain not given/ by gastric intubation. Animals were sacrificed from 2 to 480 min after administration and blood concentrations were measured. The Lewis Structure will have 4 Chlorine atoms each surrounded by 3 lone pairs, with a single bond between them and the central Chromium, which has one lone ...3 answers · 1 vote: Electron dot structure is the valence electrons are represented by dots placed around the ... Lewis Dot Structure For Aluminium Oxide April 16th, 2019 - Lewis Dot Structure For Aluminium Oxide pdf Free Download Here Lewis diagrams are electron dot pictures which give an excellent account of the and then draw their Lewis Dot Structure Oxide ion Barium ion Draw the Lewis structure for each of the following K2s lewis dot structure No IP ... A step-by-step explanation of how to draw the Lewis dot structure for Ba (Barium). I show you where Barium is on the periodic table and how to determine how...
What is the Lewis dot structure for Ba3N2? Barium nitride is a "salt like" nitride- which means that he bonding is best considered as ionic. the lewis dot is your method of showing ions Ba2+ ion...
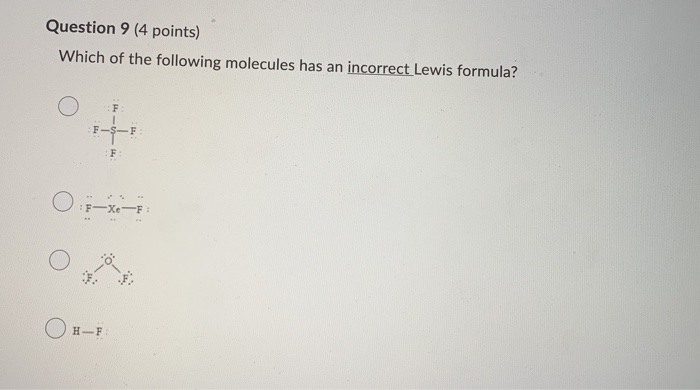
Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. Don't forget to show brackets and charge on your LDS for ions!
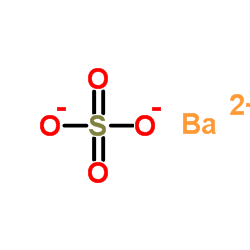
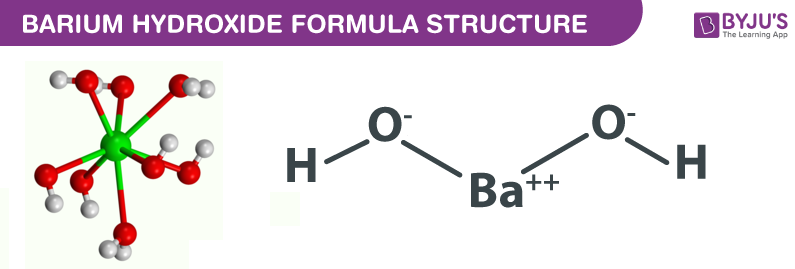
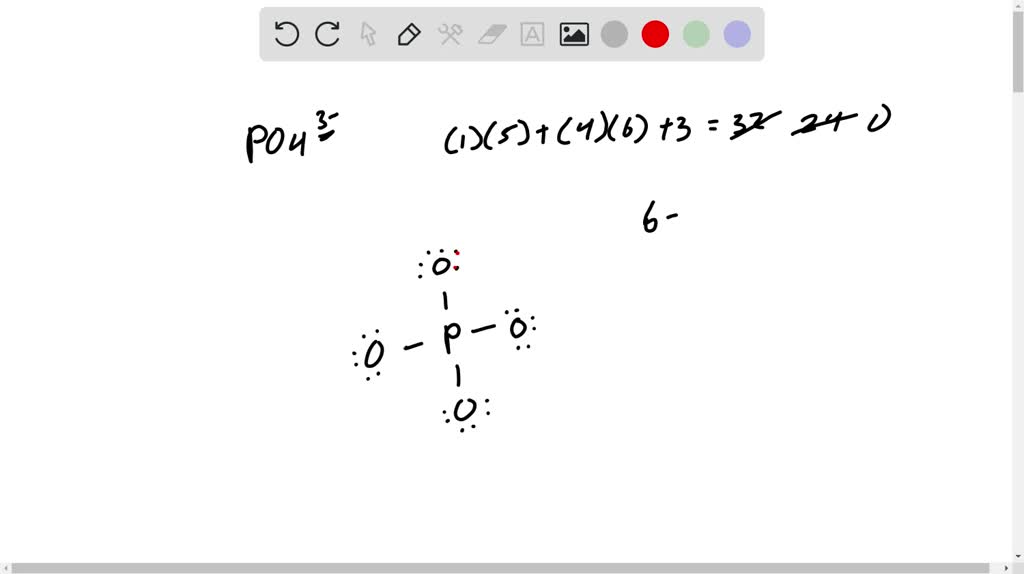
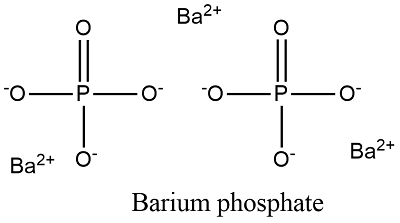
The barium phosphate constitutes the 3 cations and 2 anions. The three cations are provided by the barium atom whereas the two anions are provided by anion. The cationic form of barium is Ba2+ whereas the anionic form of the phosphate is represented as PO43-.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Barium phosphide (Ba3P2) Tribarium diphosphide.
What is the Lewis dot structure for barium ion? - Answers new www.answers.com. What is the Lewis dot structure for Ba3N2? Barium nitride is a "salt like" nitride- which means that he bonding is best considered as ionic. the lewis dot is your method of showing ions Ba2+ ion...
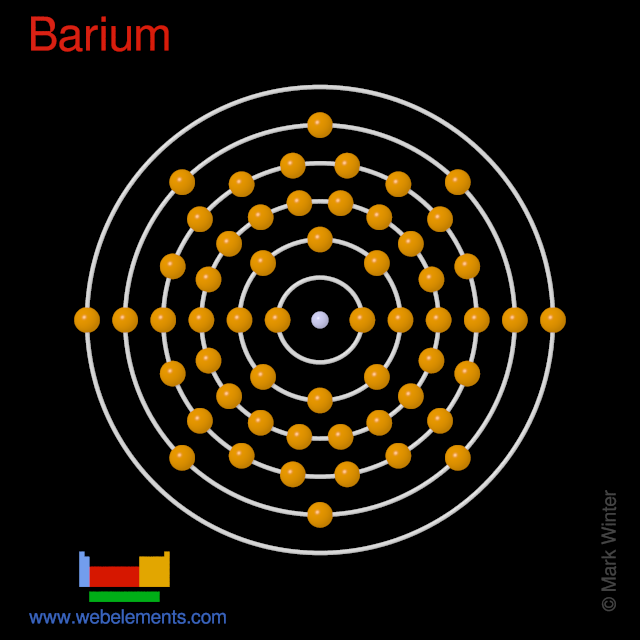
Barium has 6 electron shells with an equal sequence of electrons in each; the progression is: 2 - 8 - 18 - 18 - 8 - 2. The electron shells are therefore progressively equal in thickness and number of particles from inner to outer shells. Read in-depth answer here. Similarly, you may ask, how many electrons does barium have?
Everything you want about barium chloride lewis dot diagram will be provided by Bartendery. All information about barium chloride lewis dot diagram will always be updated with the latest, accurate.
Here are a number of highest rated Lewis Dot Diagram For Copper pictures on internet. We identified it from reliable source. Its submitted by giving out in the best field. We undertake this nice of Lewis Dot Diagram For Copper graphic could possibly be the most trending topic later than we allowance it in google lead or facebook.
A step-by-step explanation of how to draw the Ba3N2 Lewis Dot Structure.For Ba3N2 we have an ionic compound and we need to take that into account when we dra...
Lewis dot structure of the potassiumis to be drawn. Concept introduction: Lewis structure is a representation of a chemical formula of substance with valance electrons of atoms. The Lewis structures are also called electron dot structures. In the Lewis structure, electrons are denoted by dots.
Orbital Diagram For Barium The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the s,p,d,f electron configuration for Barium. Barium is an alkaline earth metal. This means that it is a group 2 element. Barium's atomic number is 56; this means that it has 56 protons in its nucleus and also.
A step-by-step explanation of how to draw the BaCl2 Lewis Dot Structure.For BaCl2 we have an ionic compound and we need to take that into account when we dra...









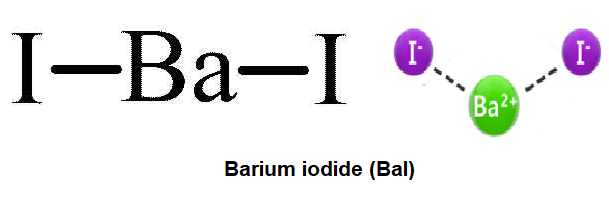




0 Response to "37 lewis dot diagram for barium"
Post a Comment