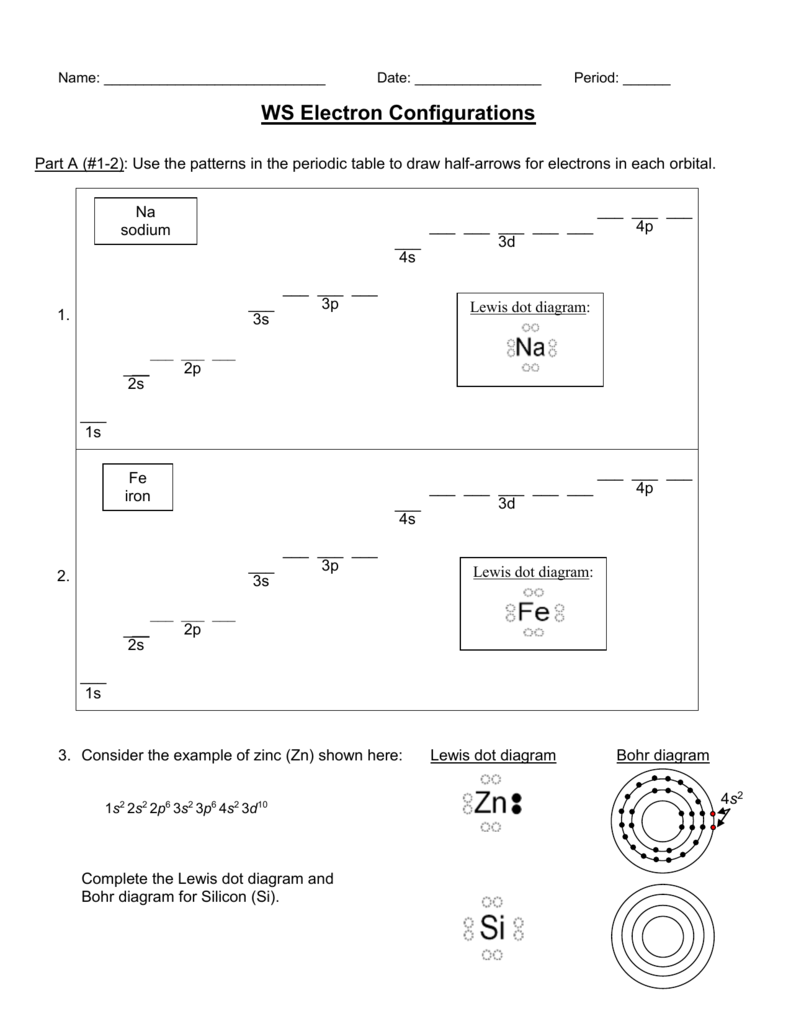
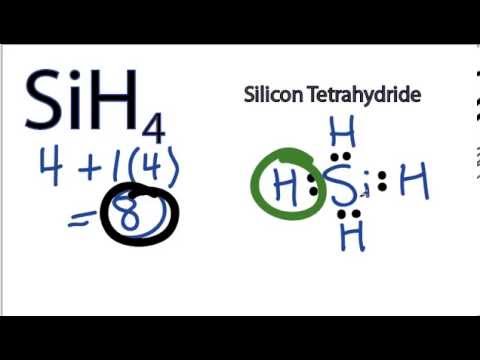
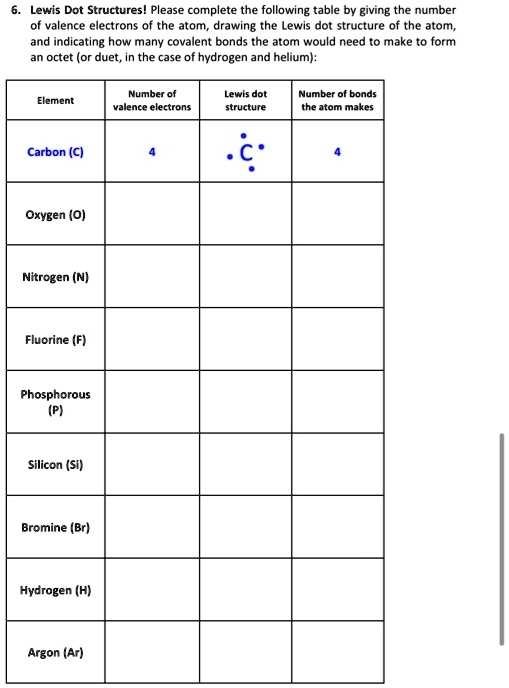
42 dot diagram for silicon
Answer (1 of 2): Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams). That said, it seems that the one followed (at least in the Western US) has you start with the right hand side and the S orbital (only the outer valence electrons!): Si: (easier to do t... Hello! Can someone please explain to me how to draw the Lewis diagrams for transition metals? I understand how to find the valence electrons based on the electron configuration. For elements 27+, they begin having more than 8 valence? Please help I am confused!
9. Draw a Lewis dot diagram for the barium atom. 10. Draw the Lewis dot diagram for the silicon atom. I I, Draw the Lewis dot diagram for the iodine atom. 12. Draw the Lewis dot diagram for the xenon atom. 13. Hypothesize: Why are noble gases considered to be non-reactive? Your group will check your answers with the instructor before moving on.
Dot diagram for silicon
What is the Lewis dot diagram for silicon? See Answer. Best Answer. Copy. It is Si with four dots. One above Si one below and one to the left and one to the right. If you don't get what I mean ... Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon ... NCI Thesaurus (NCIt) Silica is another name for the chemical compound composed of silicon and oxygen with the chemical formula SiO2, or silicon dioxide. There are many forms of silica. All silica forms are identical in chemical composition, but have different atom arrangements. Silica compounds can be divided into two groups, crystalline (or c ...
Dot diagram for silicon. Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Below is the image of the lewis dot structure of Silicon and Sulfur separately. Now let us study the steps involved to draw the Lewis structure of Silicon disulfide (SiS2): Step 1 : Note down the total number of valence electrons available to draw one molecule of silicon disulfide : It is 16 as 4 are coming from silicon atom and 6 are coming ... A step-by-step explanation of how to draw the SiCl4 Lewis Dot Structure (Silicon tetrachloride).For the SiCl4 structure use the periodic table to find the to... Construct the Lewis structure for the covalent compound silicon dioxide (SiO 2). Learn this topic by watching Lewis Dot Structures: Neutral Compounds Concept Videos All Chemistry Practice Problems Lewis Dot Structures: Neutral Compounds Practice Problems
URL: https://www.nature.com/articles/s41928-021-00687-6 DOI: 10.1038/s41928-021-00687-6 Therefore the Silicon electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 2. Video: Silicon Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to ... The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s. SiO2 Lewis Structure (Step by Step Construction) In the SiO 2 lewis Structure, the overall ratio of silicon to the oxygen atom is 1:2.The Silicon oxygen bonds are strong and keep the atoms firmly in place. Following are the steps to construct the SiO 2 Lewis Structure.. Step-1: Count the valence electrons of atoms For the SiO 2 Lewis structure, we need to figure out the number of valence ...
We review their content and use your feedback to keep the quality high. 100% (30 ratings) Transcribed image text: How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral silicon (Si) atom? How many valence electrons are in the Lewis-dot (electron dot) structure for the neutral krypton (Kr) atom? 03 O 6. Silicon Dioxide (SiO2) Lewis Structure. The Lewis structure of SiO2 is identical to the Lewis structure of CO2. The only difference is that instead of carbon, silicon is used. One silicon atom is at the middle, with two oxygen atoms bound to it in a double bond. There are no lone pairs on the central atom of the SiO2 Lewis dot structure, Describe the electron dot diagram system of representing structure. Draw electron dot diagrams for elements. Electron Dot Diagrams. lithium 1 s 2 2 s 1 1 valence electron nitrogen 1 s 2 2 s 2 2 p 3 5 valence electrons neon 1 s 2 2 s 2 2 p 6 8 valence electrons Here's fluorine. Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two. So I'm going to start with silicon here. And I know that silicon has four bonds to fluorine atoms.
In an electron dot diagram of potassium There is one dot. In an electron dot diagram of silicon there are four dots. Which element would you expect to be more reactive? 🏠 Home ⇦ Prev Question Next Question ...
Silicon dioxide (SiO2) lewis dot structure, molecular Silicon dioxide (SiO2) lewis structure. As you see in the above SiO2 lewis dot structure, we convert 2 lone pairs of electrons of each oxygen atom to a covalent bond. So, both atoms (silicon and oxygen) have 8 electrons in their valence shell. Hence we got our best and
The silicon atoms bond to the four oxygen atoms in a way which is also similar to carbon in diamond, a tetrahedral (triangular-based pyramid) structure. Which is the Lewis dot structure for silicon Si )? Since it is in Group 4 it will have 4 valence electrons.
There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...
A step-by-step explanation of how to draw the Lewis dot structure for Si (Silicon). I show you where Silicon is on the periodic table and how to determine h...
List of Atoms for 1 & 2: Silicon, Boron, Phosphorous, Oxygen, and Hydrogen 1. Draw the Lewis Dot Diagram for each of the atoms above in a neutral state. 2. Draw the Lewis Dot Diagram for each of the atoms above in a positive and negative state. Be sure to include the notation for polarity. 3. Draw the Lewis Dot Diagram for a water molecule. 4.
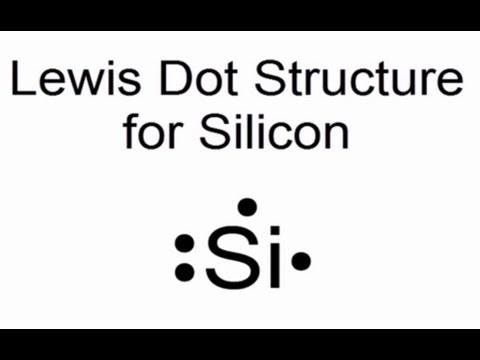
Electron dot diagram of a Silicon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Silicon, we got to know, it has only 4 valence electrons. So, just represent the 4 valence electrons around the Silicon atom as a dot.
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
Lewis Dot form of gold would be Au sign for gold with single dot. Lewis dot layout for neon has a set of electrons on each side of the neon icon, Ne, for a total of 8 electrons. Asked in Chemistry, Lewis dot layout for Li?A Lewis structure is a structural representation of particle where dots are utilized to reveal electron placement around atoms.
Q: In Lewis structure for BRF2 (Make Br central atom). H O 3 4 O5 0 7 O 8 0 9 O 10 6 H O 3 4 O5 0 7 O 8 0 9 O 10 6 A: As we know, Br and I both are 17th group elements.
Silicon is in Group 14 (sometimes called Group IV or 4). Since it is in Group 4 it will have 4 valence electrons. When you draw the Lewis structure for Silicon ...2 answers · 1 vote: Apparently there are different schools of thought on this (How to Draw Electron Dot Diagrams ...
Silicon Oxide Lewis Dot Structure by LakeView Chemistry - February 15, 2013
Electron dot structure - valence electrons are represented by dots placed around the chemical symbol. Electrons are placed up to two on each side of the elemental symbol for a maximum of eight, which is the number of electrons in a filled s and p shell.
Silicon tetrachloride (SiCl4) lewis dot structure, molecular geometry, polar or non-polar, hybridization Home > Chemistry Article > SiCl4 lewis structure and its molecular geometry Silicon tetrachloride is an inorganic compound that appears as a colorless liquid with a pungent odor having the chemical formula SiCl4.
NCI Thesaurus (NCIt) Silica is another name for the chemical compound composed of silicon and oxygen with the chemical formula SiO2, or silicon dioxide. There are many forms of silica. All silica forms are identical in chemical composition, but have different atom arrangements. Silica compounds can be divided into two groups, crystalline (or c ...
Silicon is in group 14 and period 3 of the periodic table and has four valence electrons in its Lewis structure. The four valence electrons means that silicon can bond in a way similar to carbon ...
What is the Lewis dot diagram for silicon? See Answer. Best Answer. Copy. It is Si with four dots. One above Si one below and one to the left and one to the right. If you don't get what I mean ...





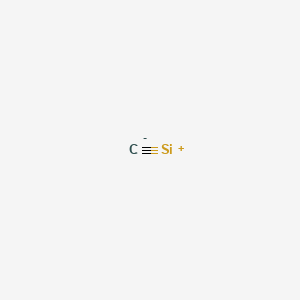

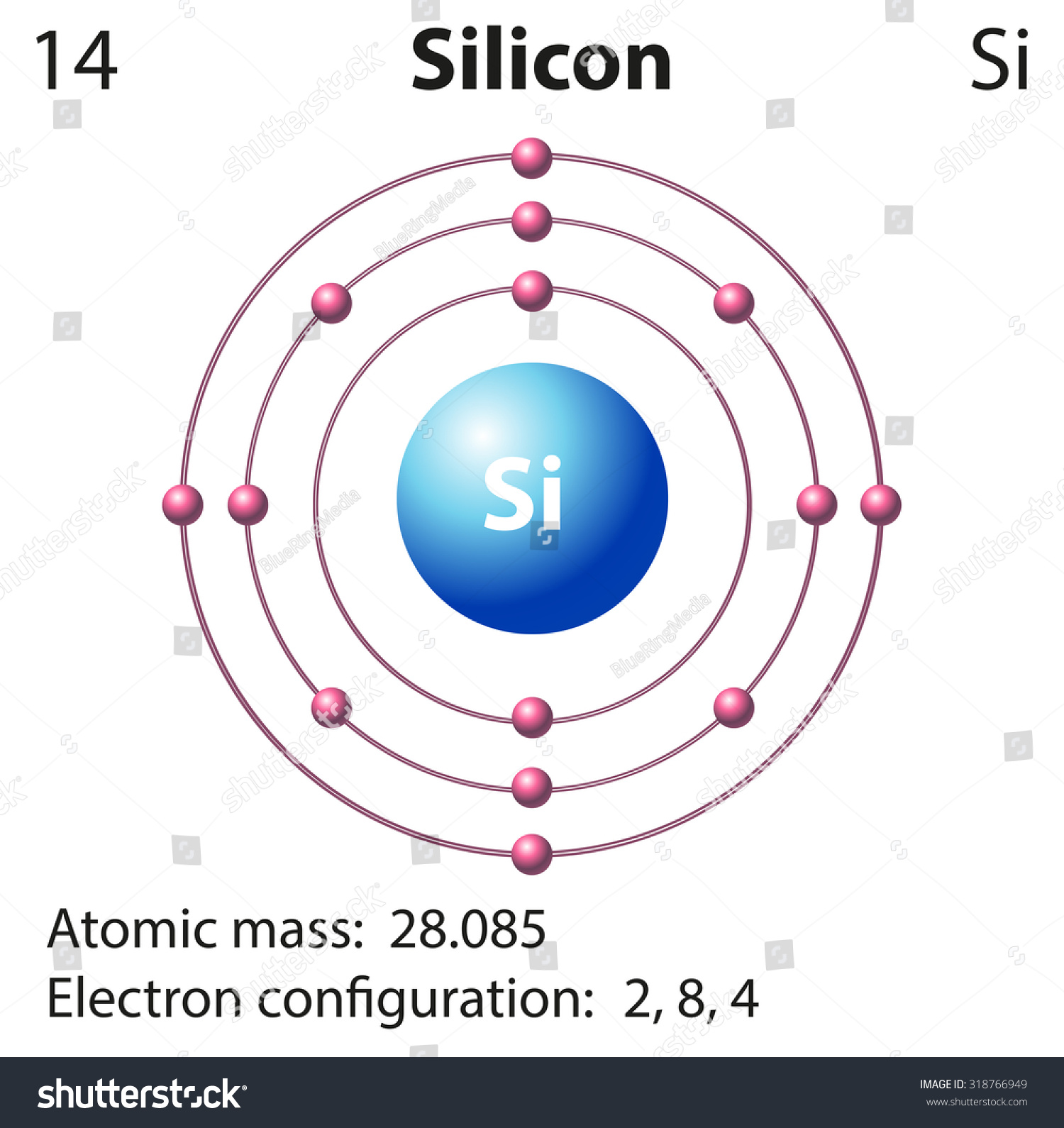








0 Response to "42 dot diagram for silicon"
Post a Comment