42 the partial (valence-level) orbital diagram for a 3+ ion is shown below.
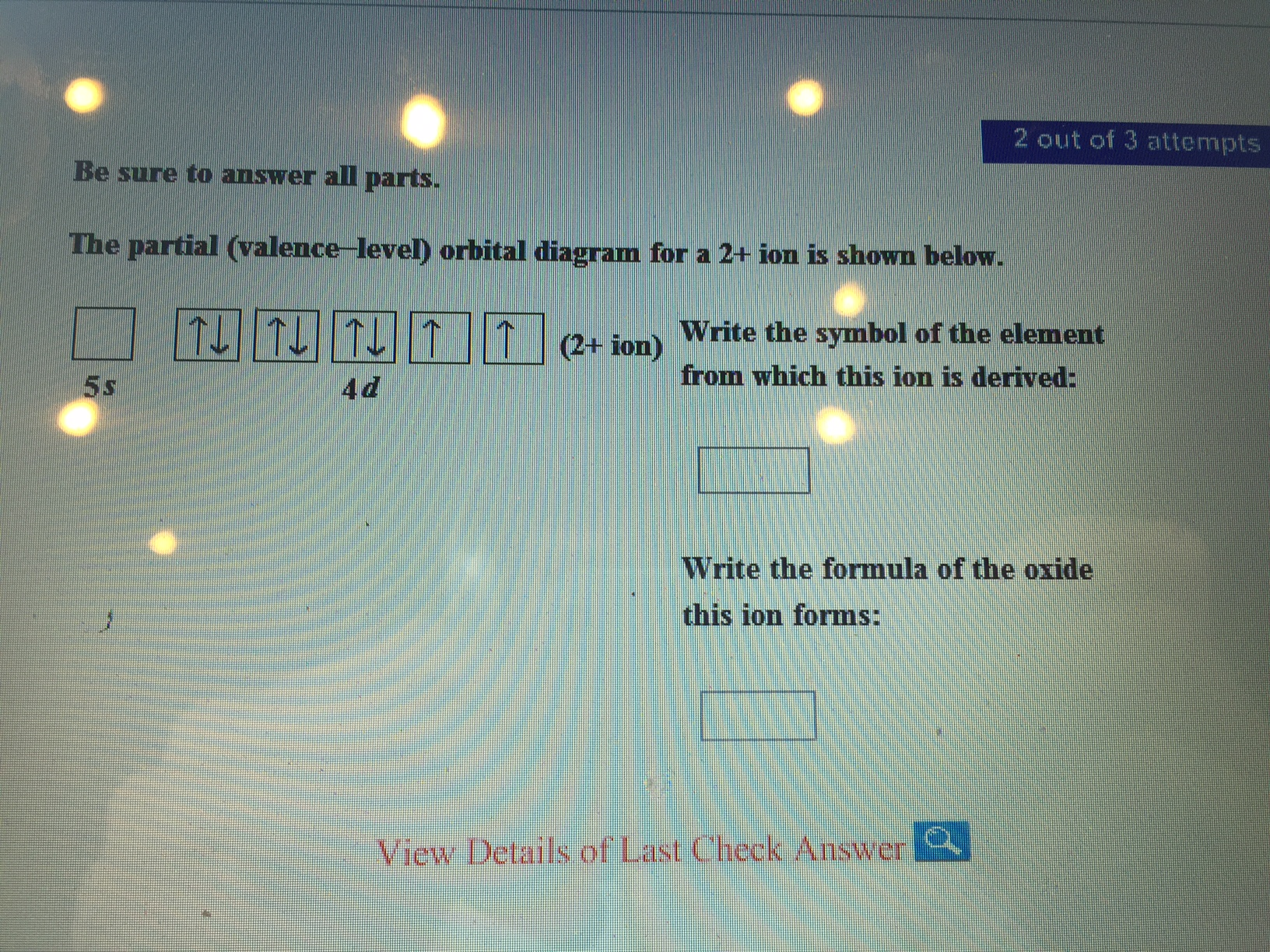
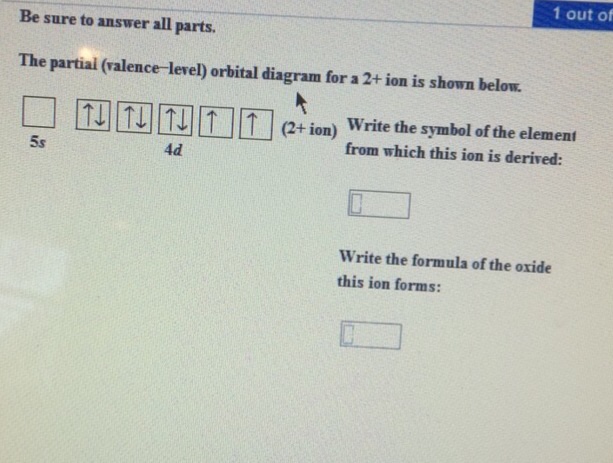
What is this element?!? The partial (valence level) orbital diagram for a 2+ ion is shown below... Then it shows a box with no arrows for the 5s orbital (aka no electrons) then it shows the 4d orbital with five boxes the first 3 boxes have an up and down arrow in each box (indicating the electrons spin (spin up spin down)) and the last 2 boxes only have spin up arrows. Sodium Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, and 3s. The Sodium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining one electron in 3s orbital. Orbital diagram for a ground-state electron configuration of Sodium atom is shown below-.
Solution Manual Brady Chemistry 6TH Edition PDF | PDF | Ion ... There are several forms of glucose, C6H12O6. Shown below are the chain form and one of the cyclic forms. Chain form. Cyclic form (-D-glucopyranose) 3.65. Binary compounds, such as CCl4 contain two elements only. A diatomic substance is composed of molecules having two atoms, such as HCl or N2. In the latter, the two atoms may or may not be the ...
The partial (valence-level) orbital diagram for a 3+ ion is shown below.
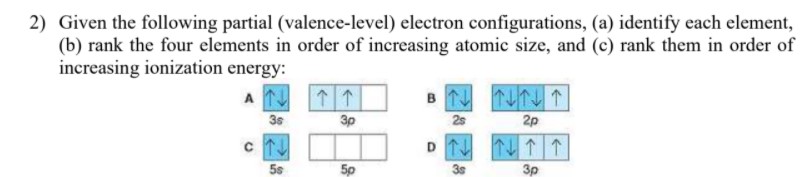
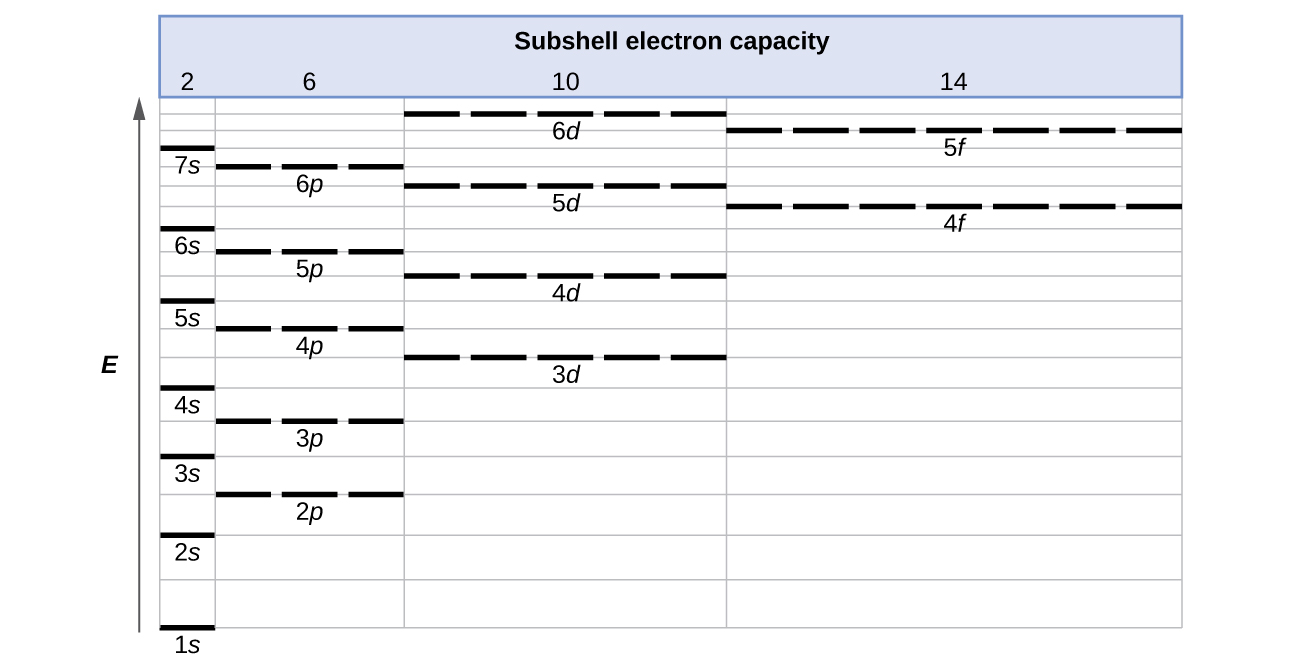
Write the electronic configurations of the ... - Clutch Prep Q. Draw the orbital diagram for the valence shell of the following atom:(e) Ru Q. Draw the orbital diagram for the valence shell of the following atom:(d) Sb Q. Partial (valence-level) electron configurations for an ion is shown below:Identify the element from which the ion is derived, and write the formula... 39 partial energy level diagram for hydrogen - Diagram For You The Lyman (ultraviolet) series of spectral lines corresponds to electron transitions from higher energy levels to level n = 1. Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion. SOLVED:Partial (valence-level) electron configurations for ... First, it's looking at this diagram. We have energy increasing as you move up the diagram. So this means that as you are adding in elections, you go from one s two to s 22 p to three s, 23 p two for us and so on. And the inverse is actually true when you're removing electrons to create an ion. So your move electrons from the highest orbital's ...
The partial (valence-level) orbital diagram for a 3+ ion is shown below.. Chemistry C2HW2 Flashcards - Quizlet a. the n = 3 shell has no f subshell b. there are three p orbitals in every shell of an atom except the n = 1 shell c. all s orbitals have spherical shapes d. each d subshell has five d orbitals e. the energies of subshell in the shells (energy levels) of a hydrogen atom vary as s < p < d, etc. Chem C2HW-B Flashcards - Quizlet a) The 1s orbital in H is more stable than the 1s orbital in He+ b) The 2s orbital in He atom is less stable than the 2s orbital in He+ c) The 2s subshell in Li is more stable than the 2p subshell in Li a. a) only b. a) and b) only c. a) and c) only d. c) only e. None of these Solved The partial (valence-level) orbital diagram for a 3 ... The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the formula of the oxide this ion forms: Question: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Answered: From the partial (valence-level)… | bartleby Solution for From the partial (valence-level) orbital diagram, write the ground-state electron configuration and group number. 2p 1 2s 11 Condensed Electron…
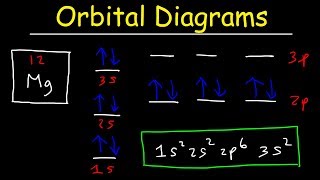
PDF Molecular Orbital Approach to Bonding M.O.Energy Level Diagram for A2 (A = Li, Be) Li2 Only two valence electrons, i.e. σs 2σ*s 0. Bond order = 1. Diamagnetic Li2 exists in gas phase over metallic lithium. "Be2" σ s 2σ* s 2 B o ndr e= 0 - t b i g energy, so molecule does not exist. Beryllium in gas phase is monatomic. Use Aufbau, Pauli, Hund - just as in filling atomic orbitals Magnesium Orbital diagram, Electron configuration, and ... The orbital diagram for Magnesium is drawn with 4 orbitals. The orbitals are 1s, 2s, 2p, and 3s. The Magnesium orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, and the remaining two electrons in 3s orbital. Orbital diagram for a ground-state electron configuration of Magnesium atom ... Answer in General Chemistry for Dawson #175590 The partial (valence-level) orbital diagram for a 3+ ion is shown below.__ 4s (no arrows) __ __ __ _ 3. How many types of isotopes do you have? 4. When potassium chlorate is heated, it decomposes into potassium, chlorine and oxygen. How many gram; 5. 1. Calculate the potential for the titration of 40.0 mL of 0.10 M Fe2+ with 5.0, 10.0, 20.0 and ... Essay Fountain - Custom Essay Writing Service - 24/7 ... When it comes to finding the best specialist for your paper there are 3 categories of specialist that we have to look at; Best available This refers to a group of writers who are good at academic writing, have great writing skills but are new in our team of writers.
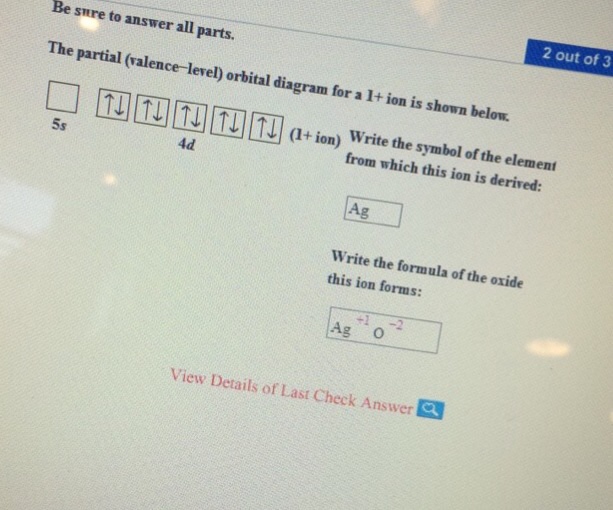
Solved The partial (valence-level) orbital diagram for a 2 ... The partial (valence-level) orbital diagram for a 2+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the formula of the oxide this ion forms: Question: The partial (valence-level) orbital diagram for a 2+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the ... Draw the partial (valence-level) orbital d... | Clutch Prep Q. Draw the partial (valence-level) orbital diagram, and write the symbol, group number, and period number of the element: (a) [Ne] 3s23p5. Q. Draw a partial (valence-level) orbital diagram, and write the condensed ground-state electron configuration for each: (c) Ag. Q. Draw a partial (valence-level) orbital diagram, and write the condensed ... CHEM MIDTERM #1 (Hwk Questions) Flashcards | Quizlet Consider the following portion of the energy-level diagram for hydrogen: n = 4-0.1361 × 10-18 J n = 3-0.2420 × 10-18 J n = 2-0.5445 × 10-18 J n = 1-2.178 × 10-18 J For which of the following transitions does the light emitted have the longest wavelength? Chapter 8, Electron Configuration and Chemical ... - Numerade Draw a partial (valence-level) orbital diagram, and write the condensed ground-state electron configuration for each: $$ \begin{array}{llll}{\text { (a) Ti }} & {\text { (b) } \mathrm{Cl}} & {\text { (c) } \mathrm{V}}\end{array} ... Period 4 transition element that forms $3+$ diamagnetic ion (n) Period 4 transition element that forms $2+$ ion ...
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM 5.13 The energy level diagram for SH- is shown below. A bond order of 1 is predicted. The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the
Answered: Draw the partial (valence-level)… | bartleby Solution for Draw the partial (valence-level) orbital diagram and write the symbol, group number, and period number of the element: [Ar] 4s²3d® 3d (select) …
SOLVED:The orbital diagram that follows shows the valence ... Video Transcript. The orbital diagram that follows shows the valence electrons for a two plus ion of an element letter. A asks what is the element And so from the orbital diagram given the island with a two plus charge has six valence electrons shown here these four in these two the ion with a two plus charge.
The Electron Configuration: Ions - Chemistry ... - Clutch Prep Ions also have electron configurations (Section 7.4). Cations have fewer valence electrons, and anions have more valence electrons, respectively, than their parent atoms. For example, chloride, Cl-, has an electron configuration of 1s22s22p63s23p6, for a total of 18 electrons, compared to 17 for neutral chlorine, the element.
PDF Ruby Solutions - WPMU DEV 3. Draw the partial (valence-level) orbital diagram for each of the following, and write the group number (the "group" is the column the element's in), the penod number (the "period" is the row), and atomic symbol of the element represented. [He]2s22p4 b. c. [Ne]3s23p3 [Ar]4s23d104p4
PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Answered: Draw the partial (valence-level)… | bartleby Science Chemistry Q&A Library Draw the partial (valence-level) orbital diagram, and writethe symbol, group number, and period number of the element: (a) [He] 2s²2p⁴ (b) [Ne] 3s²3p³.
How to Do Orbital Diagrams - Sciencing The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals.
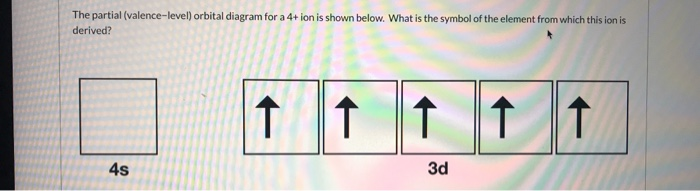
Answer in General Chemistry for Dawson #175587 The partial (valence-level) orbital diagram for a 3+ ion is shown below. __ 4s (no arrows) __ __ __ __ __ 3d (two arrows in the first box, up and down) (One up arrows in the rest of the boxes, 6 arrows total) Write the symbol of the element from which this ion is derived. Write the formula of the oxide this ion forms. The answer is not Fe or Cr.
Solved The partial (valence-level) orbital diagram for a ... The partial (valence-level) orbital diagram for a 3-ion is shown below. What is the symbol of the element from which this ion is derived? 个 4s 3d ND Cr TI OV Mn Co ; Question: The partial (valence-level) orbital diagram for a 3-ion is shown below. What is the symbol of the element from which this ion is derived? 个 4s 3d ND Cr TI OV Mn Co
Answered: Draw the partial (valence-level)… | bartleby Draw the partial (valence-level) orbital diagram, and write the symbol, group number, and period number of the element: (a) [Kr] 5s24d10 (b) [Ar] 4s23d 8. Start your trial now! First week only $4.99! arrow_forward.
Aluminum Orbital diagram, Electron configuration, and ... The orbitals are 1s, 2s, 2p, 3s, and 3p. The Aluminum orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, the six electrons in 2p orbital, the two electrons in 3s orbital, and the remaining one in 3p orbital. Orbital diagram for a ground-state electron configuration of an Aluminum atom is shown below-.
SOLVED:Partial (valence-level) electron configurations for ... First, it's looking at this diagram. We have energy increasing as you move up the diagram. So this means that as you are adding in elections, you go from one s two to s 22 p to three s, 23 p two for us and so on. And the inverse is actually true when you're removing electrons to create an ion. So your move electrons from the highest orbital's ...
39 partial energy level diagram for hydrogen - Diagram For You The Lyman (ultraviolet) series of spectral lines corresponds to electron transitions from higher energy levels to level n = 1. Energy level diagrams for IONS Atoms with 1, 2, or 3 valence electrons lose them to form 1+, 2+ or 3+ ions respectively. naming metallic ions - the full name of the atom is followed by the word ion.
Write the electronic configurations of the ... - Clutch Prep Q. Draw the orbital diagram for the valence shell of the following atom:(e) Ru Q. Draw the orbital diagram for the valence shell of the following atom:(d) Sb Q. Partial (valence-level) electron configurations for an ion is shown below:Identify the element from which the ion is derived, and write the formula...
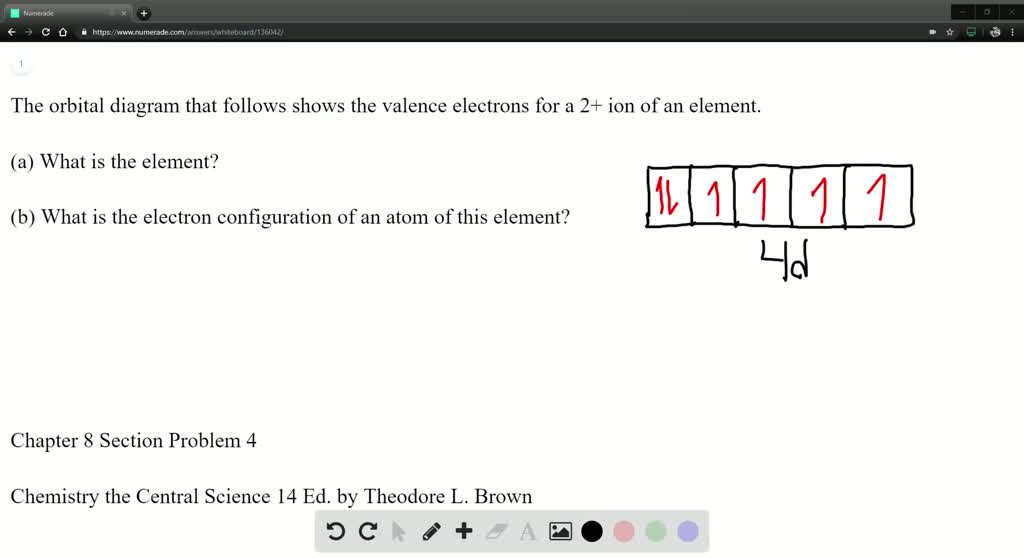
the orbital diagram that follows shows the valence electrons for a 2 ion of an element a what is the
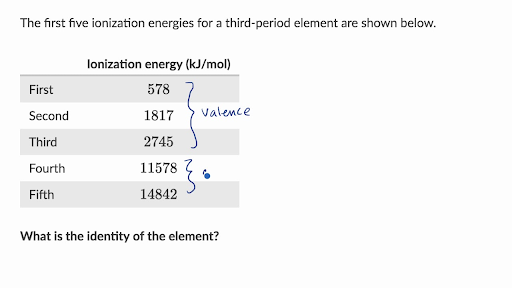
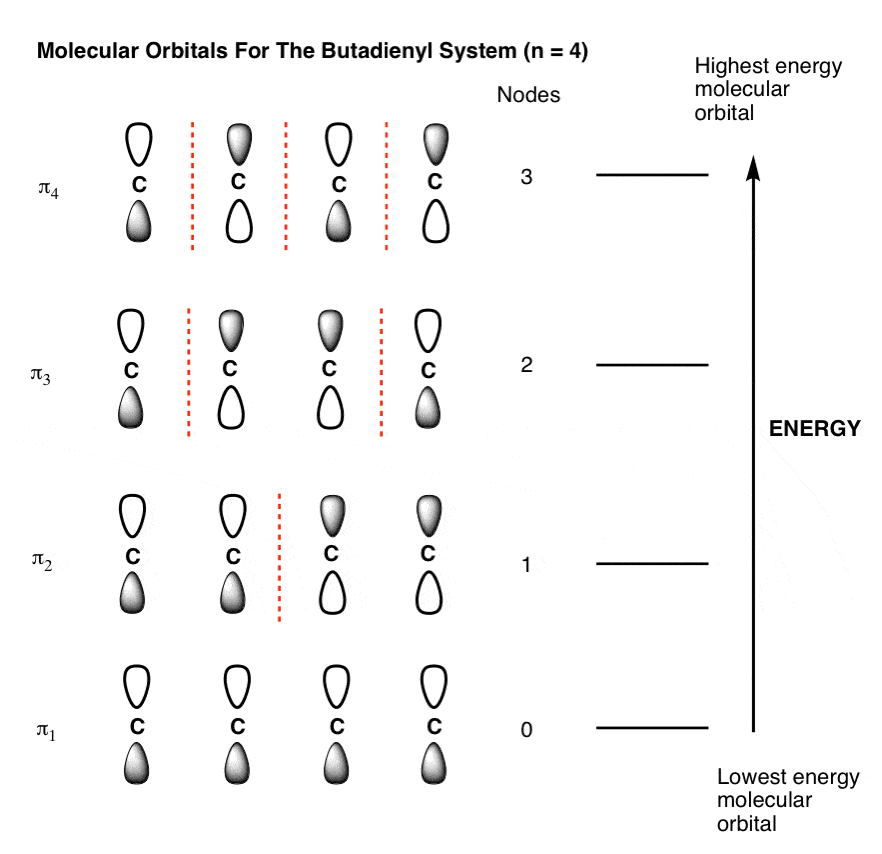
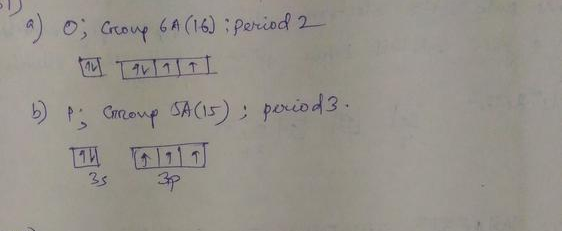

















0 Response to "42 the partial (valence-level) orbital diagram for a 3+ ion is shown below."
Post a Comment