42 Salt Water Phase Diagram
PDF Phase Diagram for CO2 - columbia.edu Phase Diagram for CO2. Phase Diagram for H2O. The Liquid State • Vapor pressure no•S teciasfneru •Viscosity •Adhesive/cohesive forces • Capillary action ... - Reclamation of brackish/salt water - Sewage treatment -Electrodialysis - Many biological/ecological processes. Osmosis/Osmotic Pressure. Osmosis/Osmotic Pressure PDF Phase Diagrams of Some Sodium and Potassium Salts In Light ... 6. 2 Salt hydrates If the solid phase is an anhydrous salt the solubility dif ference depends only on isotopic effects in the solution. If the solid phase is a hydrate it will contain H^O if it is in equilibrium with ordinary water and D?0 if it is in equilibrium with heavy water. In the latter case it may be termed a deuterate. The
The salt-water system phase diagram and its application ... Paperback. Pub Date: 2005 05 of Pages: 269 in Publisher: Tianjin University Press. salt-water system phase diagram and its application system about the principles of the phase diagram. draw two to five yuan salt-water system phase diagram phase diagram understanding of the calculation of the phase diagram and the phase diagram of the experimental method. application the Pizer electrolyte ...

Salt water phase diagram
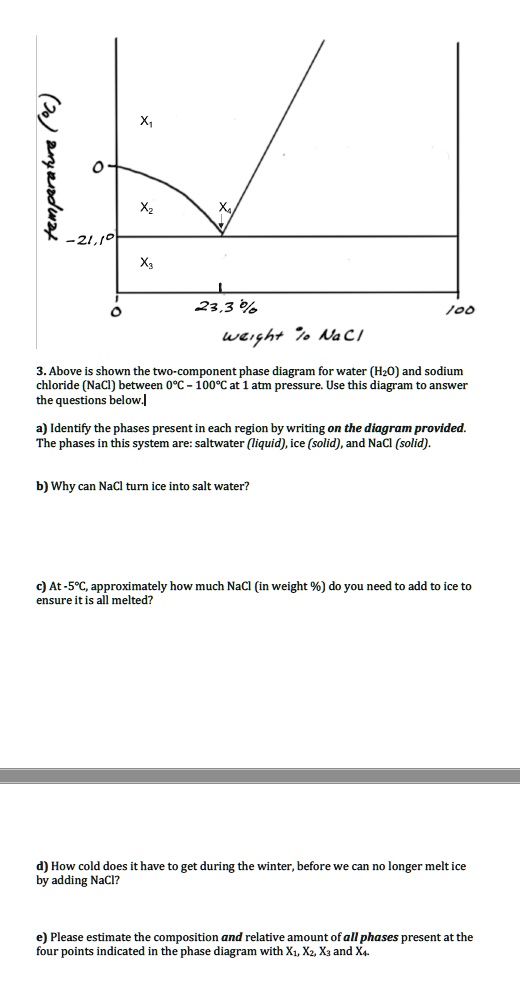
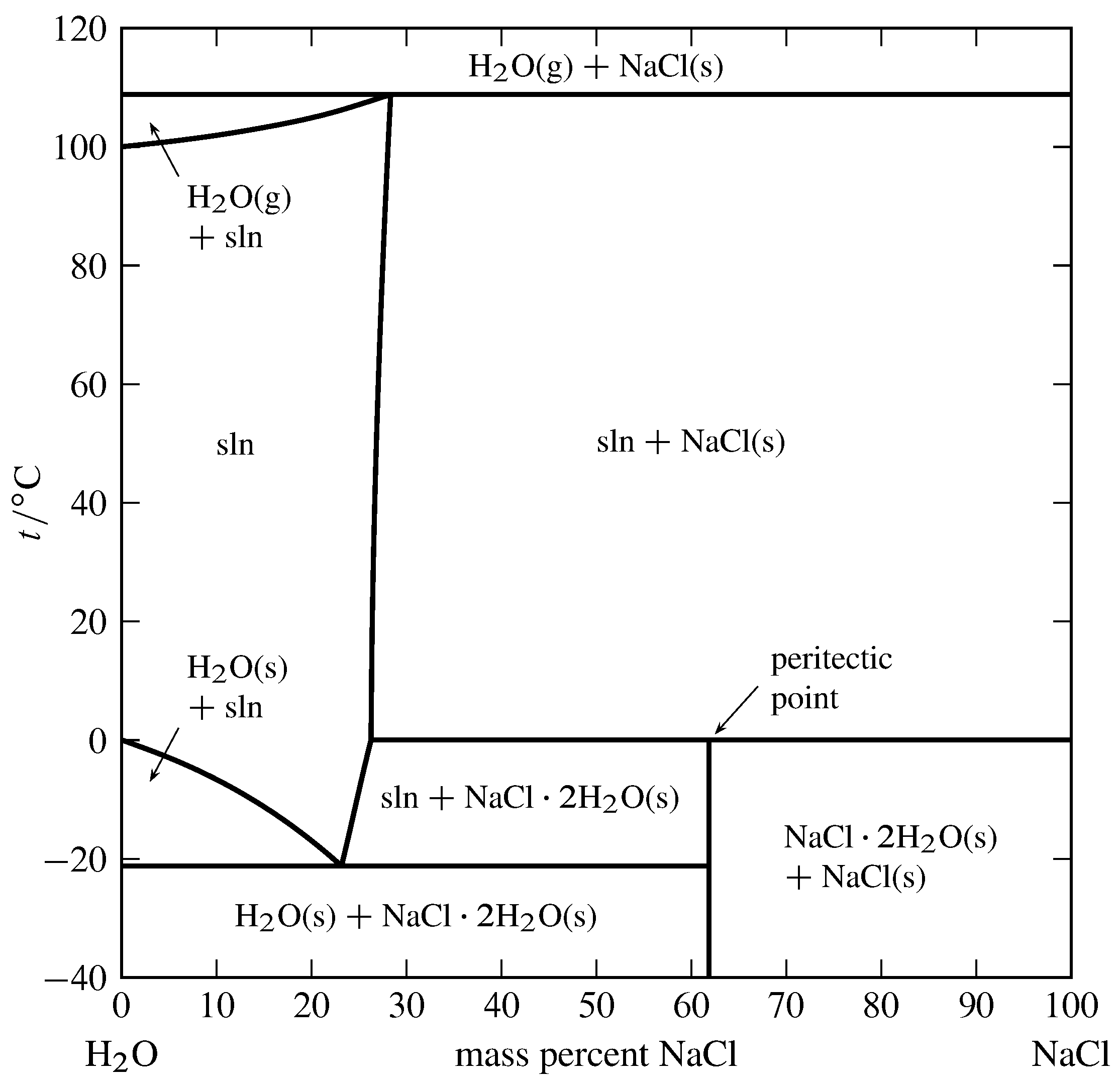
PDF Properties of solutions - UPM Solubility and phase diagram Water can only dissolve up to 26.4%wt of NaCl at 15 ºC, slightly increasing with temperature; see the phase diagram is presented in Fig. 1. The interest is just on liquid solutions, since the components do not mix in the solid state, and the amount of salt vapours can be neglected below say 1000 ºC. Understanding the Water-in-Salt to Salt-in-Water ... Understanding the Water-in-Salt to Salt-in-Water Characteristics across the Zinc Chloride : Water Phase Diagram Shelby B. Pillai Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695-8204, United States Solved This question pertains to the salt-water phase ... Transcribed image text: 2. This question pertains to the salt-water phase diagram shown below: Liquid (brine) Salt Ice Temperature (°C) Liquid (brine) Temperature (°F) Liquid (brine) Ice + Salt 20 NECE-300 1 0 HO 10090 20 30 Composition (wt%) (a) 100 g of salt is added to a beaker containing 750 g of water at 5°C and sufficiently mixed to dissolve all of the salt.
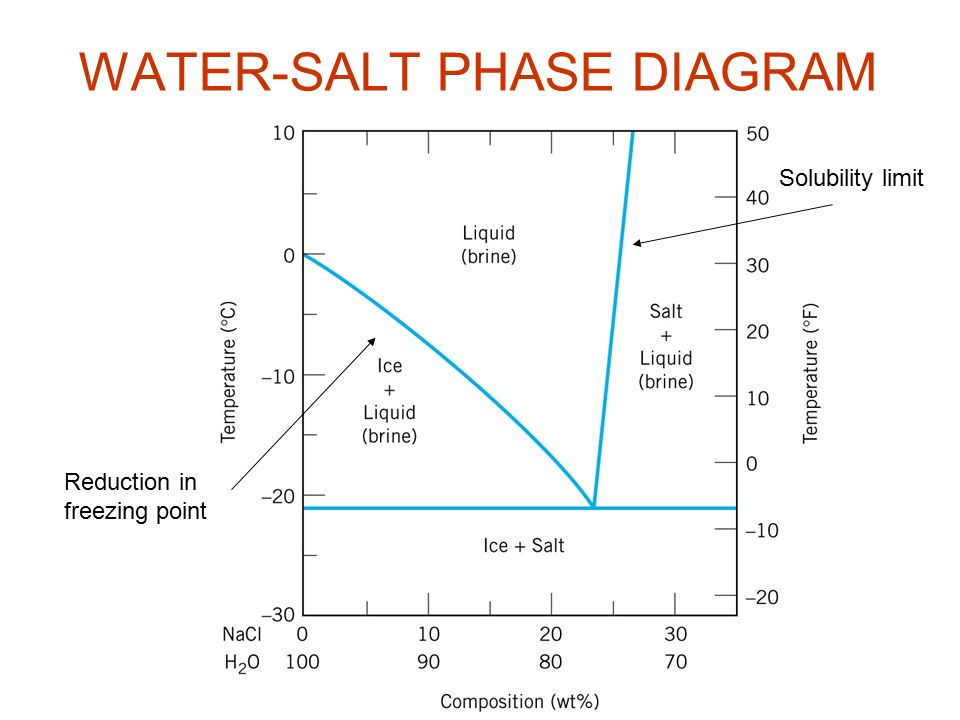
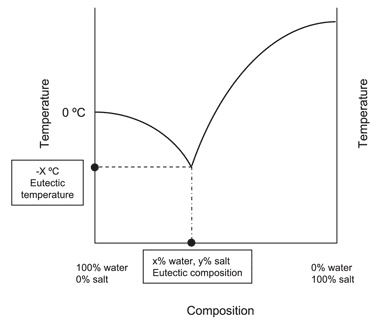
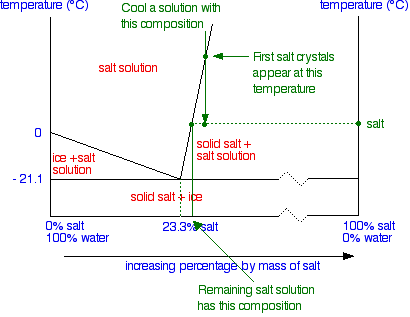
Salt water phase diagram. A typical phase diagram of a binary salt-water system ... A typical phase diagram of a salt-water system shows that the EFC process can be represented by the paths A B E or C D E in Fig. 1. The salt concentration of the feed solution can be either higher ... PDF Chapter 9: Phase Diagrams - Florida International University Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e. Chapter 9 - 3 • Components : thermodynamics - Phase diagram of water - Physics Stack ... Exactly. For instance, try googling the phase diagram for salt water and you will see a very different diagram depending on the amount of salt. I am guessing your hope was that you could separate a mixture into its constituents and use the phase diagrams of each constituent. But that is unfortunately not the case. Phase Diagrams Explained - Demystifying Ice Cream ... The phase diagram of salt (NaCl) and water. Source. Let us walk through two examples: When the concentration of salt is 5% and the temperature is -10°C we end up in the red area: ice + salt water. This means that there is both salt water and ice in this mixture.
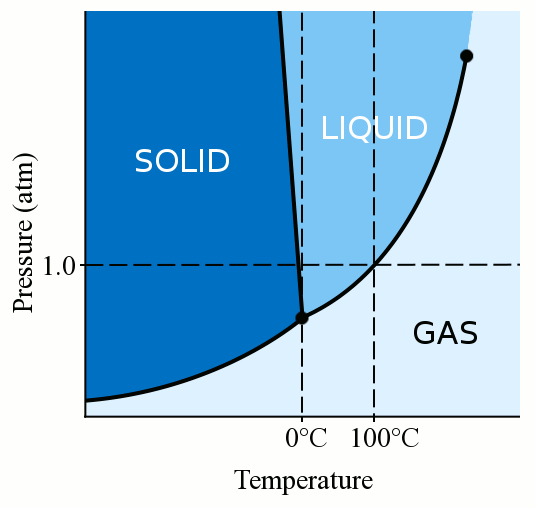
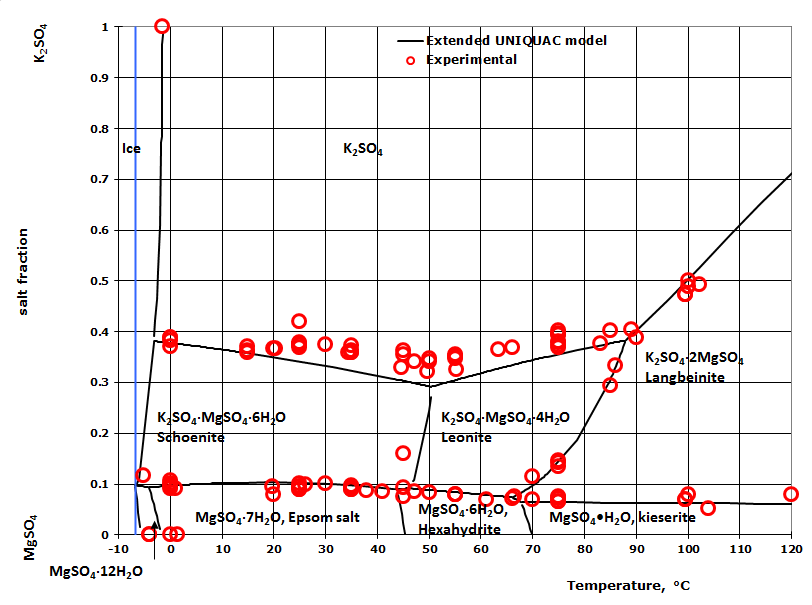
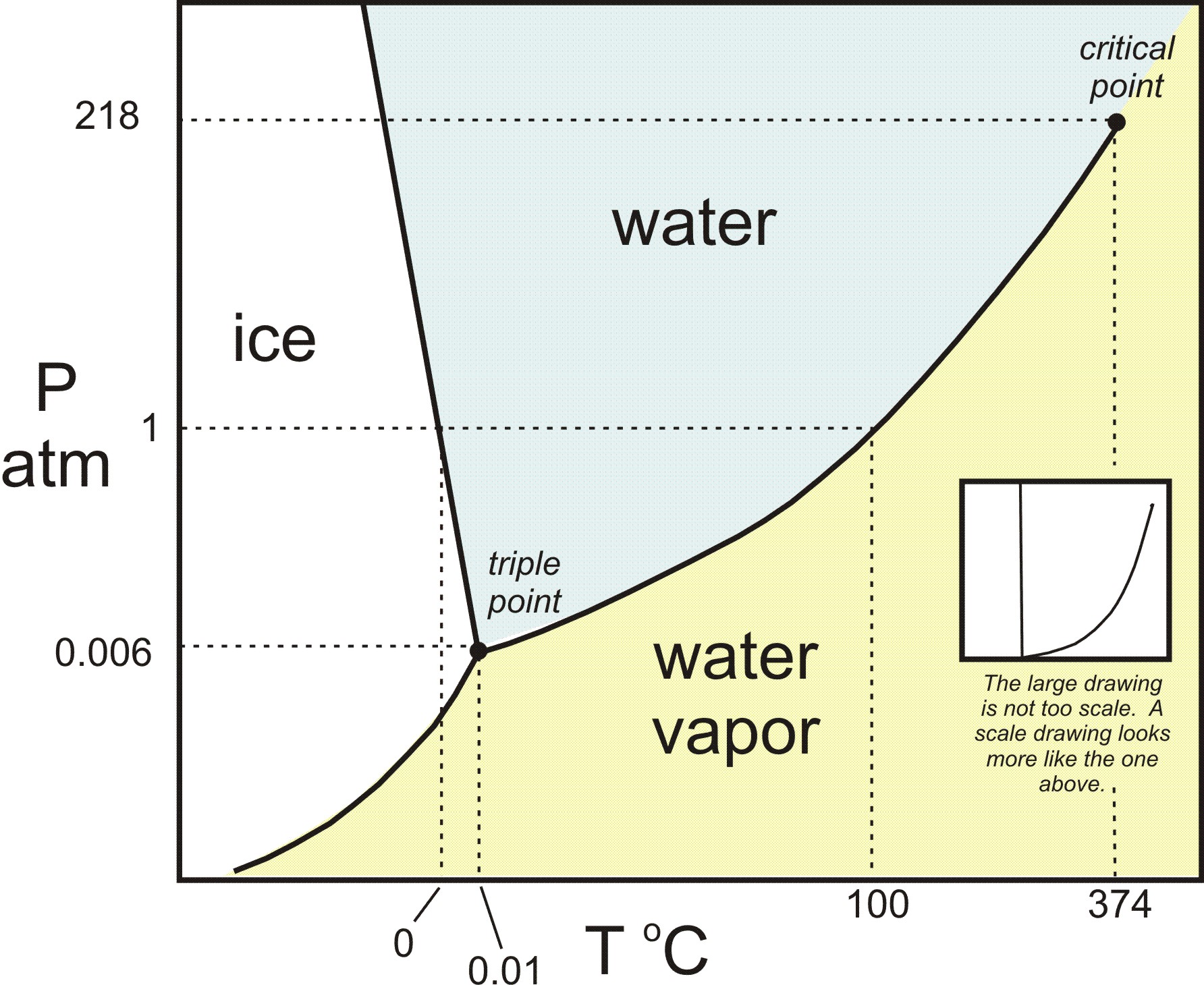
Phase Diagrams - Chemistry - University of Hawaiʻi Determining the State of Water Using the phase diagram for water given in , determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa. Solution PDF Phase diagram of water - Columbia University Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. WATER Covers ~ 70% of the earth's surface Life on earth depends on water Water is a "universal" solvent Easily polluted; hard to purify. Stable-Phase Diagram of the Quaternary Water-Salt System ... The stable-phase diagram of the quaternary system K +, Rb +, Cs + //SO 42- -H 2 O at T = 323.2 K consists of four invariant points, nine univariant curves, and six crystallization regions. Solid-liquid Phase Diagrams: Salt Solution This page looks at the phase diagram for mixtures of salt and water - how the diagram is built up, and how to interpret it. It includes a brief discussion of solubility curves. Important: This page is only really designed to be an introduction to the topic suitable for courses for 16 -18 year olds ...
Saline water - Wikipedia Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride).The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around ... PDF Quick Determination of Water Phase Salt Content of Smoked Fish COMPUTE WATER PHASE SALT CONTENT Water Phase Salt can be calculated by using either percent or grams of salt and moisture from the analysis. 100 % % % X Salt Moisture Salt WPS + = or X100 gSalt gMoisture gSalt WPS + = Example: Using the example above where a10 gram sample of smoked fish was found to have 60% moisture and 2.88% salt (0.288 g ... PDF Chem 253, UC, Berkeley Reading: West 11-12 Phase Diagram Most phase diagrams at 1 atm Reading: West 11-12 Chem 253, UC, Berkeley Phases Homogeneous portion of the system with uniform physical and chemical characteristics Salt - water Salt NaCl A difference in either physical or chemical properties constitutes a phase Water and ice FCC and BCC polymorphic forms of an element PDF Transition Temperatures of the Hydrates of Na2SO4 ... - NIST 2.1 Salt transitions The hydrated crystal is a distinct phase of the water: salt binary system. The phase transition which occurs when a hydrated inorganic salt changes its state of hydration may be either congruent or incongruent depending on whether one or two solid phases are involved. In the former, the hy
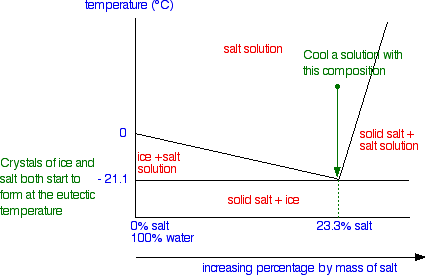
Liquid-Solid Phase Diagrams: Salt Solutions - Chemistry ... The labeled areas in the phase diagram. These areas all show what you would see if you had a particular mixture of salt and water at a given temperature. For example, if the temperature was below -21.1°C, you would always see a mixture of solid salt and ice. There would never be any liquid whatever proportions of salt and water you had.
PDF Binary Phase Diagrams - Clark Science Center temperatures and composition by constructing a phase diagram using temperature as the vertical axis and using the compositions H2O and NaCl to define a horizontal composition axis . At low temperatures, the "melt" in the H2O-NaCl system is salt water or "brine." Brines may be more or less salty depending on the ratio of NaCl to H2O, i.e ...
Salts/Salt Mixtures - Saltwiki The phase diagram shows the different hydration phases that the salt can form. Different lines will show their stability areas. The different DRH for the hydrates are shown by the thicker drawn lines, and they describe the equilibrium between the crystalline salt, the saturated solution and the water vapor in the air.
Phase Diagram of Salt Water - Technische Fakultät Phase diagram of salt water. "Ice" means solid and rather pure H 2 O with almost no dissolved salt in this case. Never mind the NaCl end! NaCl · 2 H 2 O means that you have a rock salt crystal with water molecules as a kind of dissolved impurity (2 H 2 O molecules occupying the place of one NaCl unit).
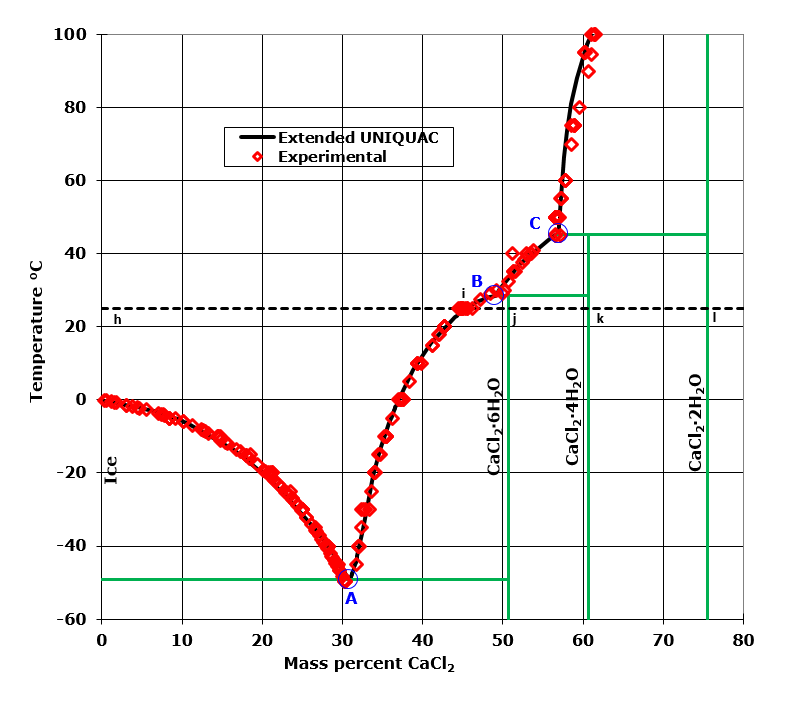
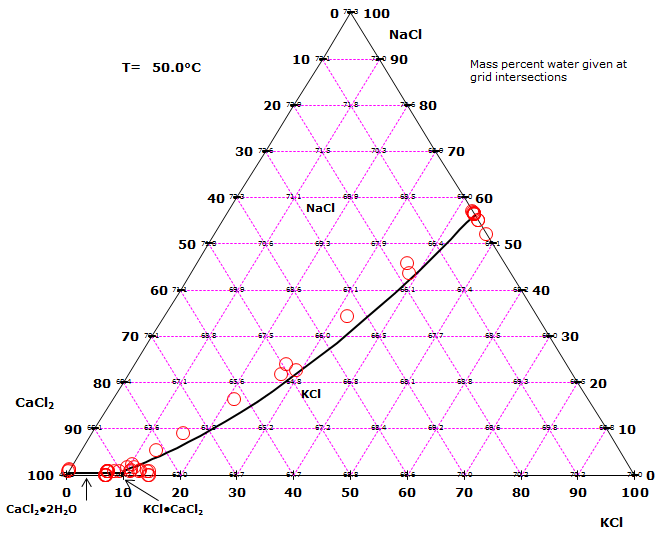
Phase diagrams for quaternary salt solutions : Phasediagram Phase diagrams for two quaternary salt solutions are shown in the tabs below. This type of phase diagrams are for example useful when designing fractional crystallization processes. The sodium chloride, potassium chloride, calcium chloride - water system.
Phase diagrams for ternary salt solutions : Phasediagram Several phase diagrams for ternary salt solutions are shown in the tabs below. This includes Partial pressures in the carbon dioxide - ammonia - water system Solubility isotherms in the following systems: Sodium nitrate - sodium sulfate - water Sodium sulfate - magnesium sulfate - water Magnesium sulfate - potassium sulfate water
FTsalt - FACT Salt Phase Diagrams (351) FTsalt - FACT Salt Phase Diagrams (351) Click on a system to display the phase diagram.
General Chemistry Online: FAQ: Solutions: Why does salt ... Phase map for salt water. Drawn from a diagram by R. E. Dickerson (Note 3) As ice begins to freeze out of the salt water, the fraction of water in the solution becomes lower and the freezing point drops further. This does not continue indefinitely, because eventually the solution will become saturated with salt.
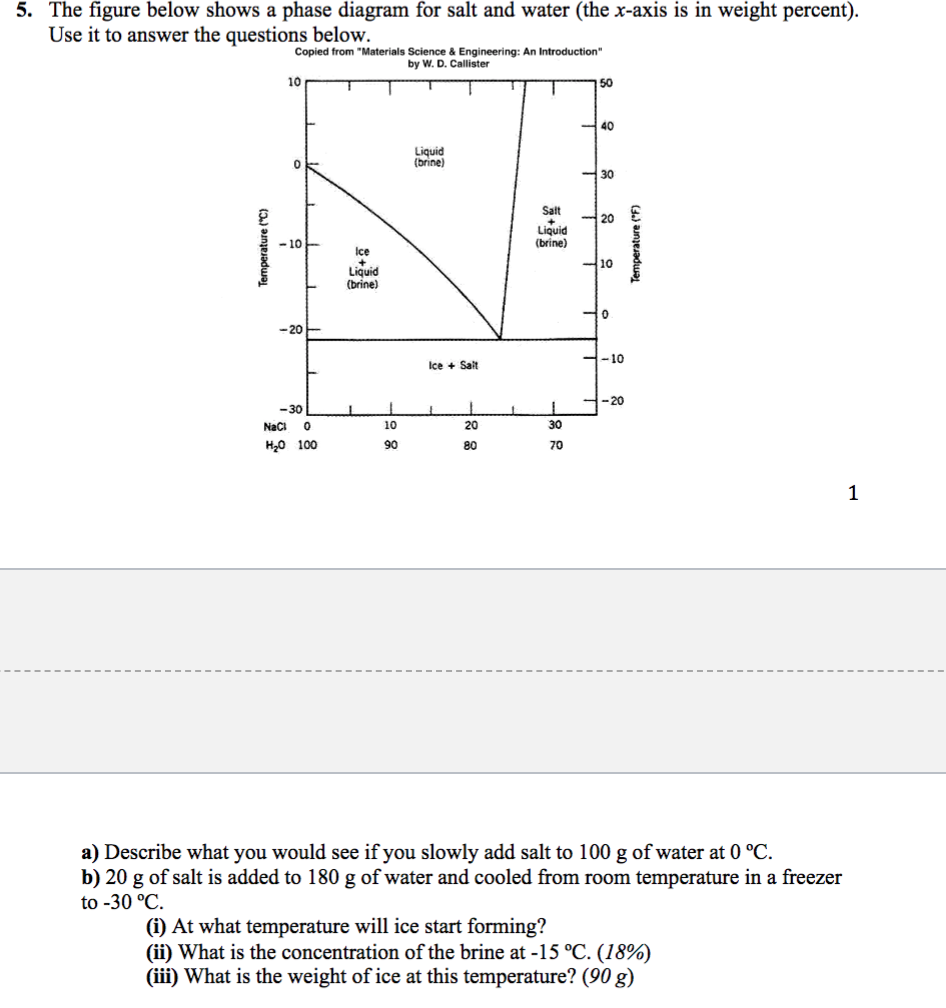
Solved Referencing the figure below of the H2O ... - Chegg Mechanical Engineering questions and answers. Referencing the figure below of the H2O-NaCl (water-salt) phase diagram: a. Explain why placing salt on ice can cause it to melt (give an example for a specific temperature and discuss the phase changes that occur as salt is added). b. If the solution is 15% salt by weight, determine the temperature ...
PDF The Influence of Inorganic Salts on the Phase Diagram and ... polymer-organic and salt-water phases. In the presented work, the phase diagrams of the water-polymer biphasic system PEG 6000-sodium citrate (C 6 H 5 O 7 Na 3)-water and the concentration effect of the sodium nitrate on the position of the binodal, on the value of the separating ability of the system were investigated.
Phase diagram of salt water. | Download Scientific Diagram Typical binary phase diagram of salt water (Fig. 3) shows the temperature-composition fraction of NaCl relationships among the different phases of the salt water. For a binary solution of 4% NaCl ...
Solved This question pertains to the salt-water phase ... Transcribed image text: 2. This question pertains to the salt-water phase diagram shown below: Liquid (brine) Salt Ice Temperature (°C) Liquid (brine) Temperature (°F) Liquid (brine) Ice + Salt 20 NECE-300 1 0 HO 10090 20 30 Composition (wt%) (a) 100 g of salt is added to a beaker containing 750 g of water at 5°C and sufficiently mixed to dissolve all of the salt.
Understanding the Water-in-Salt to Salt-in-Water ... Understanding the Water-in-Salt to Salt-in-Water Characteristics across the Zinc Chloride : Water Phase Diagram Shelby B. Pillai Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695-8204, United States
PDF Properties of solutions - UPM Solubility and phase diagram Water can only dissolve up to 26.4%wt of NaCl at 15 ºC, slightly increasing with temperature; see the phase diagram is presented in Fig. 1. The interest is just on liquid solutions, since the components do not mix in the solid state, and the amount of salt vapours can be neglected below say 1000 ºC.

![PDF] Separating NaCl and AlCl3·6H2O Crystals from Acidic ...](https://d3i71xaburhd42.cloudfront.net/36471df76e1993c8a2064532a692c9bfaba2b3b1/3-Figure1-1.png)


















0 Response to "42 Salt Water Phase Diagram"
Post a Comment