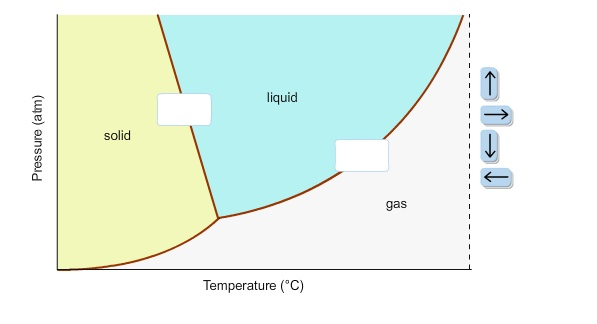
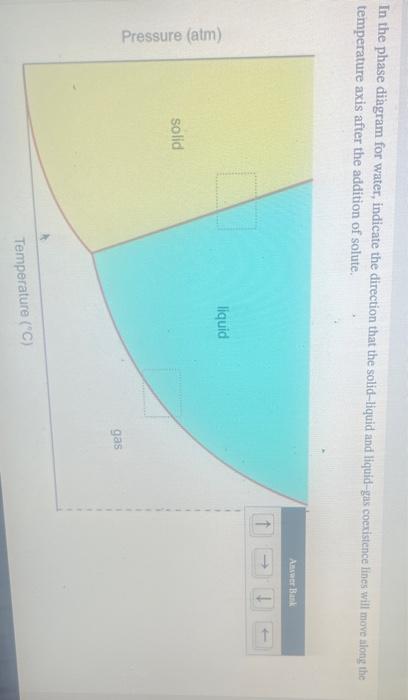
40 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
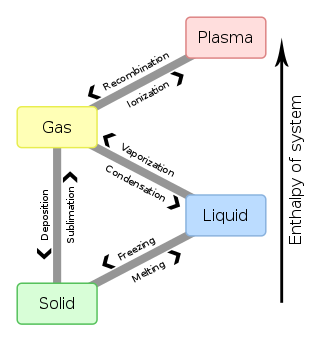
Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water. The axes correspond to the pressure and temperature. The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas . Gas - Wikipedia Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).. A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide).A gas mixture, such as air, contains a variety of pure gases.
States of Matter: Basics - Atoms | Molecules - PhET Heat, cool and compress atoms and molecules and watch as they change between solid, liquid and gas phases.
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
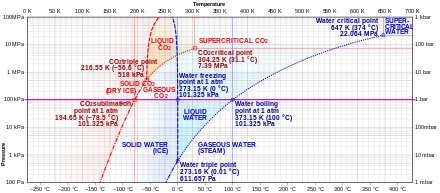
PDF Chapter 3 Thermodynamic Properties In the single-phase liquid and vapor regions the pressure decreases at fixed temperature as specific volume increases. For temperature greater than or equal to the critical temperature, there is no passage across the two-phase liquid-vapor region. Figure 3.1-2 T-v diagram for water (to scale). Figure 3.1-2 is a T-v diagram for water. For ... Phase Diagram for Water | Chemistry for Non-Majors In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water. Therefore, a pressure change has the opposite effect on those two phases. Phase (matter) - Wikipedia This unusual feature of water is related to ice having a lower density than liquid water. Increasing the pressure drives the water into the higher density phase, which causes melting. Another interesting though not unusual feature of the phase diagram is the point where the solid-liquid phase line meets the liquid-gas phase line.
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas. Answered: In the phase diagram for water,… | bartleby Science Chemistry Q&A Library In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank liquid solid gas Temperature (°C) Pressure (atm) Phase Diagrams | Chemistry - Lumen Learning A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ... Chemistry 1 Exam Flashcards - Quizlet Classify each phase change based on whether it describes a transition between a gas and a liquid, a gas and a solid, or a liquid and a solid Gas and liquid -condensation -evaporation Gas and solid -sublimation -deposition Liquid and solid -freezing -melting Label the heating curve with the phase or phases present. Assume constant pressure Page 2. CH 3–CH 3 i. 3. Use the graph to answer the ... 12.03.2022 · CH 4 c. Q. 00 Temperature {degrees C) 2) 3) 4) 6) Label the following on the phase diagram above: Solid phase, liquid phase, gas phase, triple point, critical point. Gas Properties Gas Thermodynamics Teaching Chemistry Expand 10 7 3 none 3 students will apply their knowledge of states of matter and phase changes in a game of quiz quiz trade. Chemistry. …
Guide for completing phase two environmental site ... 22.03.2016 · "contaminant" means any solid, liquid, gas, odour, heat, sound, vibration, radiation or combination of any of them resulting directly or indirectly from human activities that causes or may cause an adverse effect. For RSCs, the standards for selected contaminants are set out in the "Soil, Ground Water and Sediment Standards for Use under Part XV.1 of the … T&HE: LESSON - 19 p-V DIAGRAM, T-s DIAGRAM, p-T DIAGRAM ... 19.1. p-V DIAGRAM. 19.1.1. p-V diagram for water (solid-liquid-vapor region) If we heat ice at different vapor pressures and note down the corresponding change in volumes, the saturation state points for solid, liquid and vapor (state from which a change of phase may occur without change of pressure and temperature) for different pressures may be obtained on a p-V diagram. PDF Archived Lecture Notes #10 - Phase Equilibria and Phase ... the conditions under which the solid and liquid phases may coexist. These conditions are graphically presented in equilibrium phase diagrams, which can be experimentally determined. 2. THE ONE-COMPONENT PHASE DIAGRAM . Figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. 10.4 Phase Diagrams - Chemistry The solid-liquid curve labeled BD shows the temperatures and pressures at which ice and liquid water are in equilibrium, representing the melting/freezing points for water. Note that this curve exhibits a slight negative slope (greatly exaggerated for clarity), indicating that the melting point for water decreases slightly as pressure increases.
Polymer - Wikipedia In polymers, crystallization and melting do not suggest solid-liquid phase transitions, as in the case of water or other molecular fluids. Instead, crystallization and melting refer to the phase transitions between two solid states i.e., semi-crystalline and amorphous). Crystallization occurs above the glass-transition temperature (T g) and below the melting temperature (T m). Glass … PDF Phase diagram of water - Columbia University Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. ... Melting point - temperature at which a substance turns from solid to liquid. solid liquid gas. The three phase changes can be brought about by changes in temperature or pressure: Phase Diagrams - Purdue University When a solid is heated at constant pressure, it melts to form a liquid, which eventually boils to form a gas. Phase diagrams can be used in several ways. We can focus on the regions separated by the lines in these diagrams, and get some idea of the conditions of temperature and pressure that are most likely to produce a gas, a liquid, or a solid. Phase Diagrams - Chemistry - University of Hawaiʻi A distinct boundary between the more dense liquid and the less dense gas is clearly observed. As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water (), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor ...
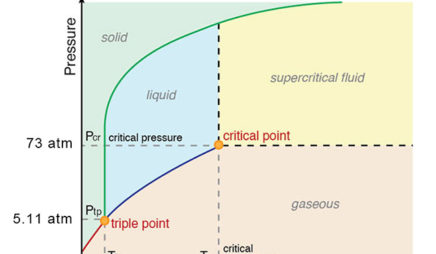
Phase Diagrams - Purdue University The phase diagram for water is shown below. The solid lines identify the temperatures and pressures at which an equilibrium exist between phases. The point at which the lines intersect represents the triple point. At the pressure and temperature of the triple point, all three phases (solid, liquid and gas) exist in equilibrium.
In this phase diagram for water, indicate the direction ... A phase diagram is a graph of pressure versus temperature depicting the solid, liquid and gaseous phases of a single substance under different conditions of temperature and pressure. In the phase diagram of water, the solid-liquid coexistence line indicates the point at which the solid converts to liquid. The temperature corresponding to
On the phase diagram, the melting point is... | Clutch Prep In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Q. Suppose a small sample of pure X is held at 190° and 0.9 atm.
In this phase diagram for water, indicate the direction ... In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Expert's Answer Solution.pdf Next Previous
Heating Curves and Phase Diagrams (M11Q2) - UW-Madison ... Figure 2. A heating curve and phase diagram for water. Solution. While anywhere along the line segment BD represents a phase change from solid to liquid, and points Y and Z are both on that line, the correct answer is D. At point Y, the phase change is occurring at the same pressure (1 atm) that was used to construct the heating curve.
Chem Sapling Hw Ch 14 Flashcards - Quizlet In this diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Solid <----- Liquid Liquid ------> Gas 10. A solution is made by dissolving 0.617 mol of nonelectrolytes solute in 889 g of benzene. Calculate the freezing point, Tf, and boiling point Tb of the solution.
Phase Diagrams - Phases of Matter and Phase Transitions 05.11.2019 · Some phase diagrams contain additional information. For example, a phase diagram for a substance that forms a crystal may contain lines that indicate the different possible crystal forms. A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. A phase diagram for an organic ...
HW2 - Homework Answers - Google Search In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. 15. Menthol is a crystalline substance with a...
Solved In the phase diagram for water, indicate ... - Chegg Science Chemistry Chemistry questions and answers In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank 1. → liquid Pressure (atm) solid gas Temperature (°C)
Fundamentals of Chemical Engineering ... - Academia.edu Fundamentals of Chemical Engineering Thermodynamics Kevin D. Dahm Rowan University Donald P. Visco Jr. University of Akron. CENGAGE Learning
Phase Diagrams - Phases of Matter and Phase Transitions A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. A phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. Mesophases are of particular interest for liquid crystal technology.
Phase Diagrams - Chemistry LibreTexts 03.05.2021 · Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to the left as the diagram for water shows. This indicates that the liquid phase is more dense than the solid phase. This phenomenon is caused by the crystal structure of the solid phase. In the solid …
OneClass: In this phase diagram for water, indicate the ... In this phase diagram for water, indicate the direction that thesolid-liquid and liquid-gas coexistence lines will move after theaddition of solute. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute.
State of Matter Questions and Answers - Study.com State of Matter Questions and Answers. Get help with your State of matter homework. Access the answers to hundreds of State of matter questions that are explained in …
This Diagram Represents A - bauservice-stemann.de 07.03.2022 · The class diagram depicts a static view of an application. This preview shows page 2 - 4 out of 9 pages. This diagram represents a process. It entails the use of short-text labels, arrows, circles and rectangles to describe data flow direction. In this case, the flow of money (green arrow in the diagram below) goes from households to firms, in ...
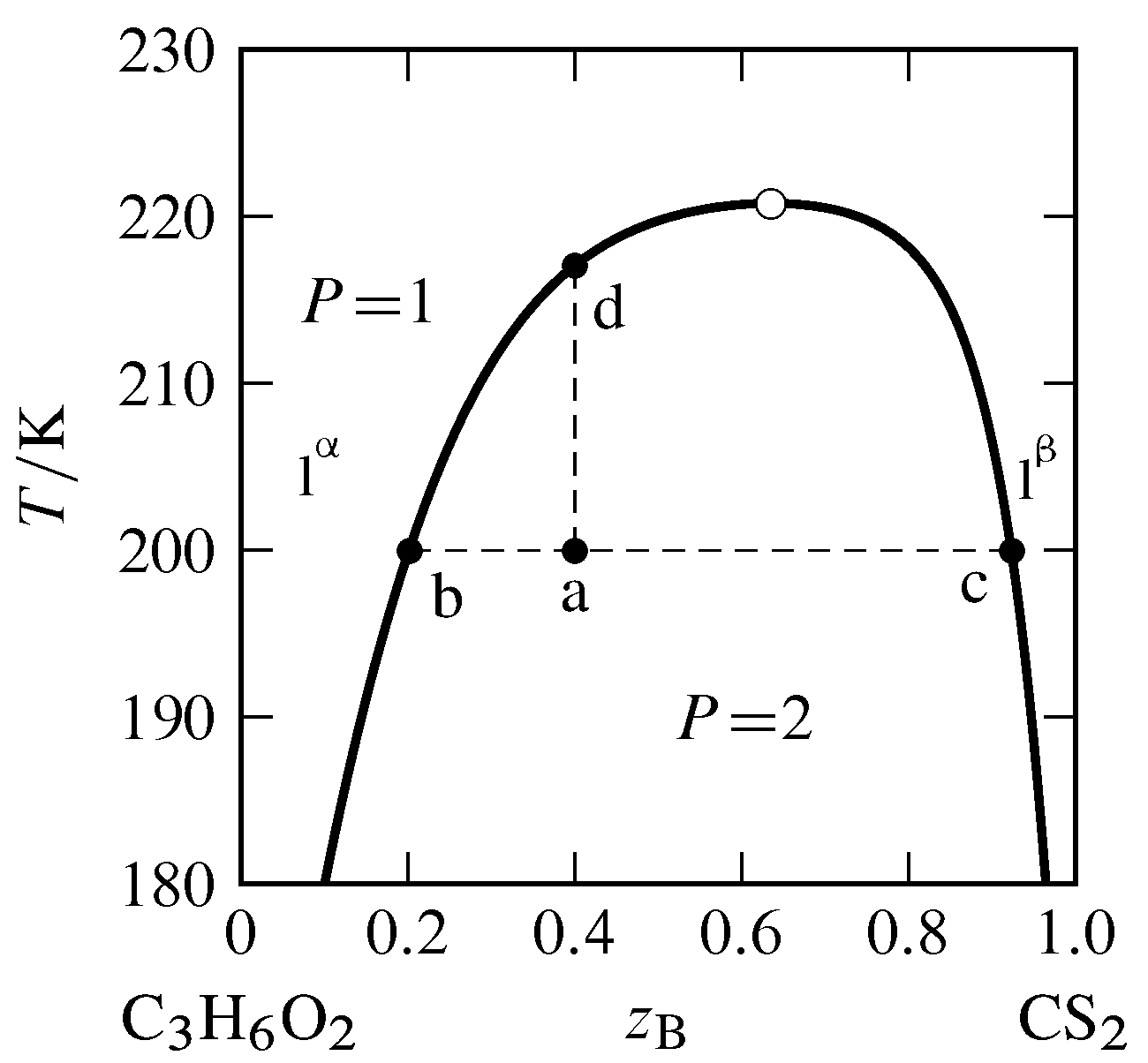
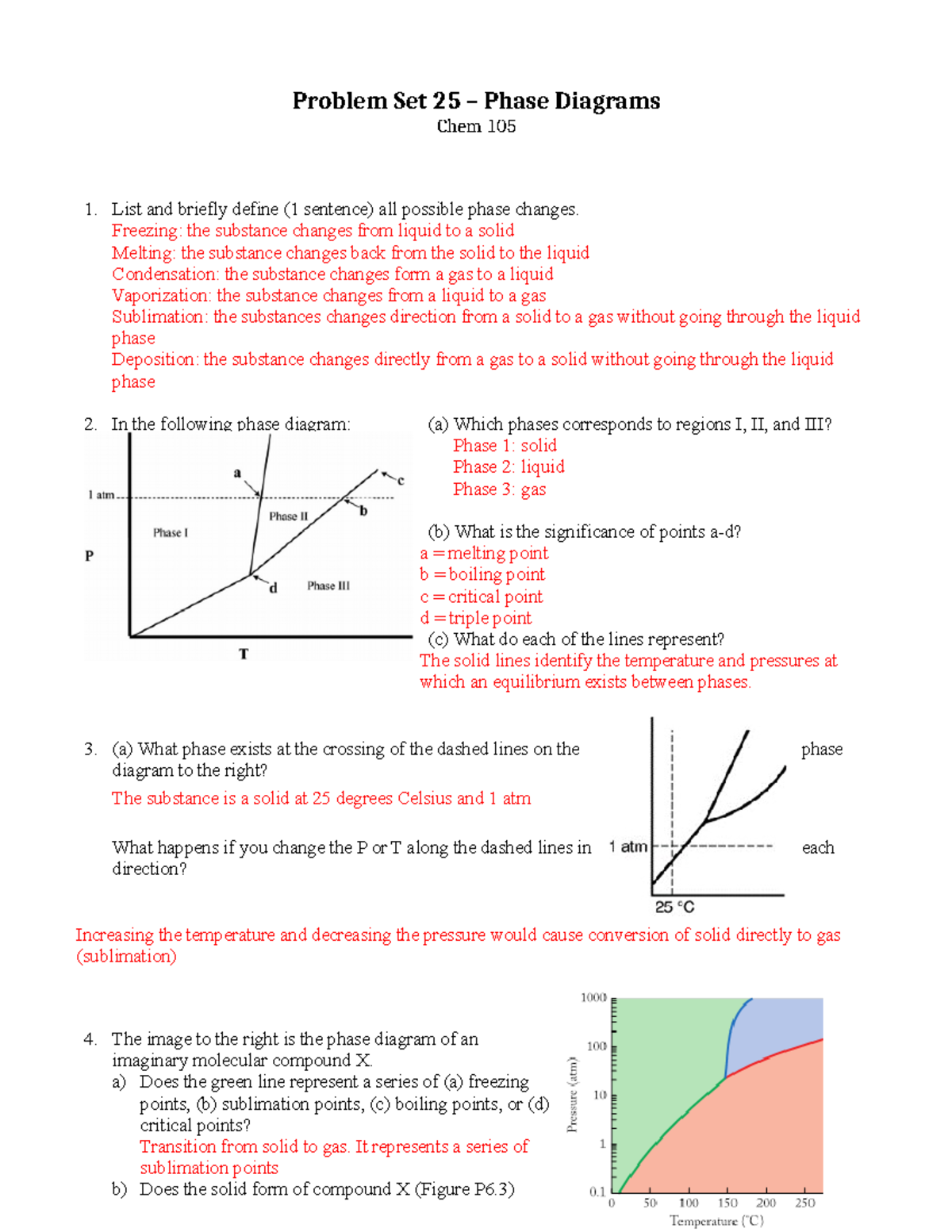
PDF Chapter 9: Phase Diagrams - Florida International University TA A 35 Co 32 CL At TA = 1320°C: Only Liquid ( L) CL = C o ( = 35 wt% Ni) At TB = 1250°C: Both α and L CL = Cliquidus ( = 32 wt% Ni here) Cα = Csolidus ( = 43 wt% Ni here) At TD = 1190°C: Only Solid ( α) Cα = Co ( = 35 wt% Ni ) Co = 35 wt% Ni Adapted from Fig. 9.3(b), Callister 7e.
In the phase diagram for water, indicate the | Chegg.com In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute, Aner Bank 1 liquid Pressure (atm) solid gas Temperature ("C)
Phase Diagrams - GitHub Pages The line that connects points A and C is the vapor pressure curve of the solid phase. Along this line, the solid is in equilibrium with the vapor phase through sublimation and deposition. Finally, point A, where the solid/liquid, liquid/gas, and solid/gas lines intersect, is the triple point
Introduction to Phase Diagrams* - ASM International Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a physically homogeneous state of matter with a given chemical composition and arrangement of atoms. The simplest examples are the three states of matter (solid, liquid, or gas) of a pure element. The solid, liquid, and gas states of a pure element obviously have the same chemical
phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
6. Phase Transitions — Introduction to Statistical Mechanics A diagram showing the stable phase of a system for every combination of macroscopic variables is called its phase diagram. Figure 6-2 shows a schematic phase diagram for water as a function of temperature and pressure. The real phase diagram is much more complicated, of course; as I said before, water actually has a huge number of different phases.
Phase (matter) - Wikipedia This unusual feature of water is related to ice having a lower density than liquid water. Increasing the pressure drives the water into the higher density phase, which causes melting. Another interesting though not unusual feature of the phase diagram is the point where the solid-liquid phase line meets the liquid-gas phase line.
Phase Diagram for Water | Chemistry for Non-Majors In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water. Therefore, a pressure change has the opposite effect on those two phases.
PDF Chapter 3 Thermodynamic Properties In the single-phase liquid and vapor regions the pressure decreases at fixed temperature as specific volume increases. For temperature greater than or equal to the critical temperature, there is no passage across the two-phase liquid-vapor region. Figure 3.1-2 T-v diagram for water (to scale). Figure 3.1-2 is a T-v diagram for water. For ...




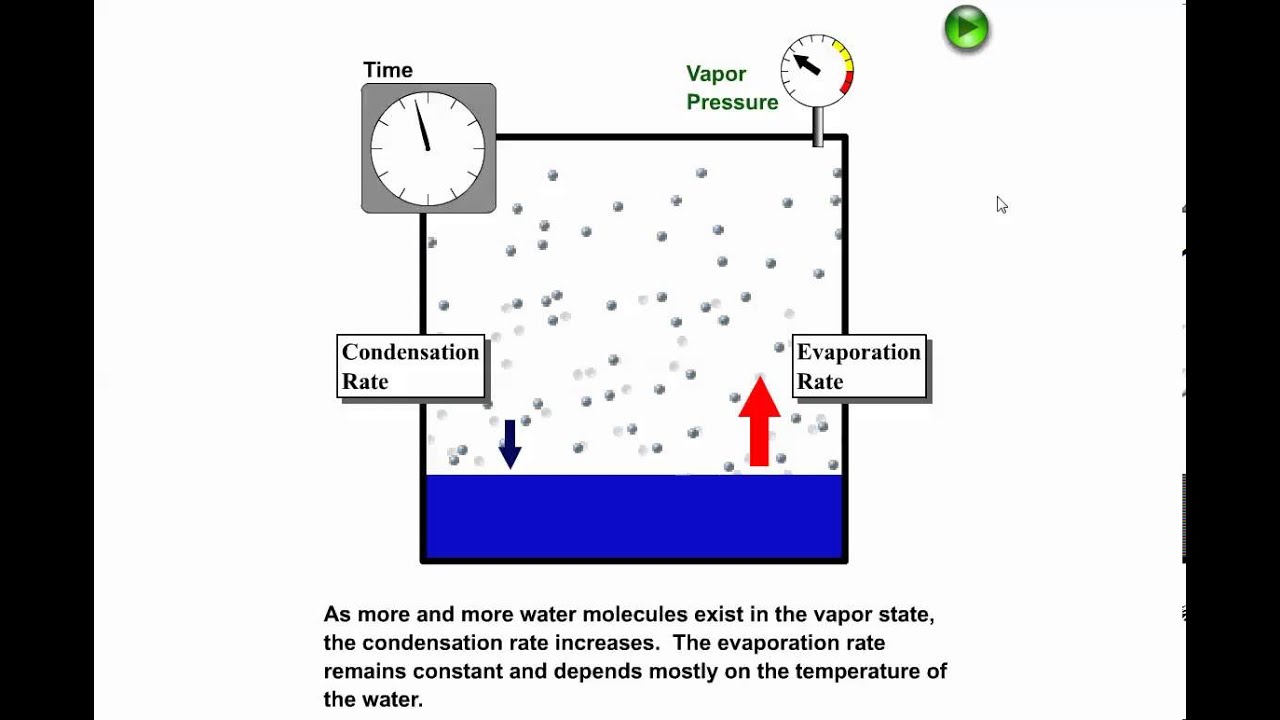
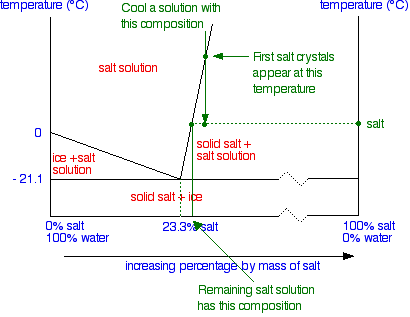
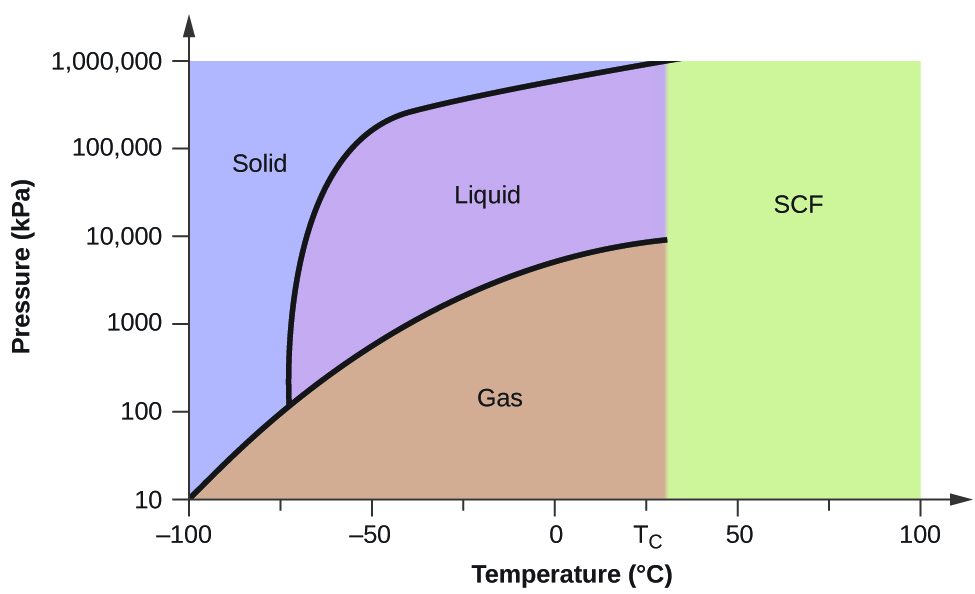
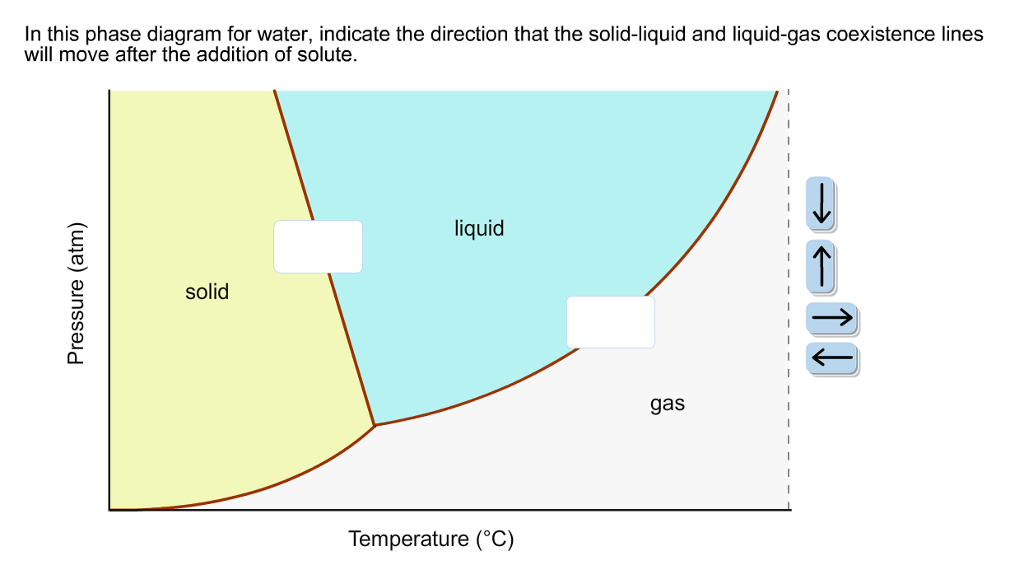
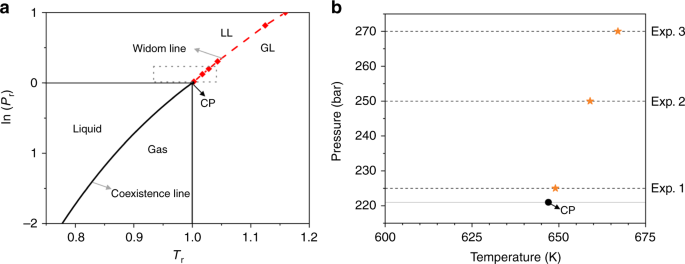

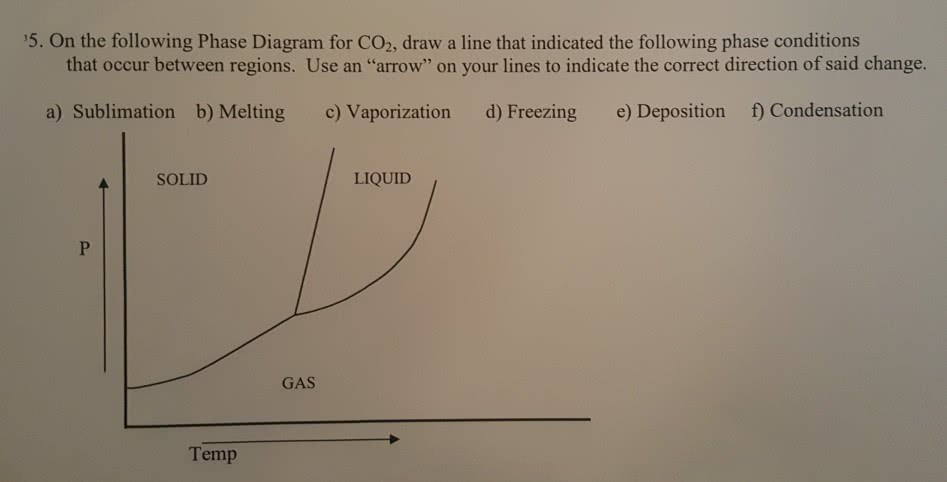
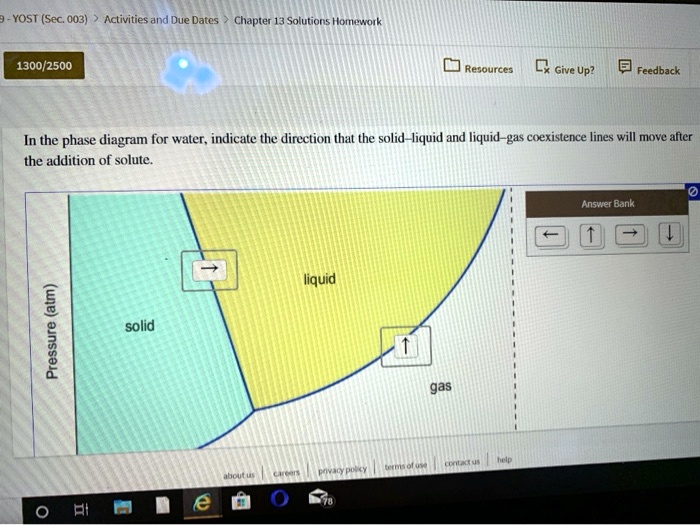
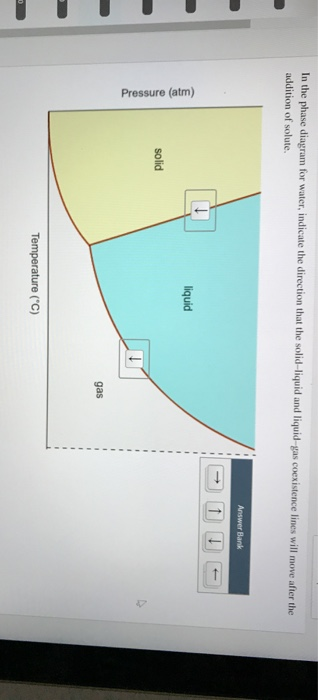








0 Response to "40 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas"
Post a Comment