40 lih molecular orbital diagram
Solved 3. Write down the Slater determinant for the lithium ... Write down the Slater determinant for the lithium hydride (LiH), the molecular orbital diagram of which is depicted below. Li2s H1s 20 Lils lơ Write down the Slater determinant for Be (1s22s2) and the first excited state of Li. The ground state of Li is 1s 2s1 4. LiH Molecular Orbital Diagram - why is the H1s AO lower ... LiH Molecular Orbital Diagram - why is the H1s AO lower than Li2s AO when Li2s has higher Zeff? 7 comments. share. save. hide. report. 100% Upvoted. This thread is archived. New comments cannot be posted and votes cannot be cast. Sort by. best. level 1. 1 year ago.
A Revisit to Molecular Orbitals in H2+, LiH, HF, and ... It is considered that the usual explanations for the molecular orbitals of H-2(+), LiH, HF, and hybridization in the conventional textbooks are liable to misunderstanding. Discover the world's ...
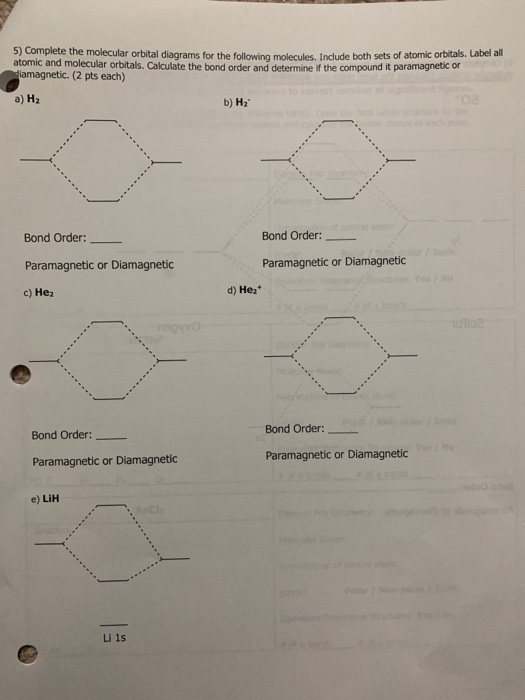
Lih molecular orbital diagram
PDF A Molecular Orbital Study of the Dipole Moment of HF, LiH ... In the ab initio molecular orbital calculations, the widely used 6-311G** basis sets are adopted throughout this study. 7,8 We use the MCSCF wave functions in order to treat the dissociation limit correctly. We use CASSCF within the configuration of 2 electrons in 2 orbitals for LiH, full-CI for PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Molecular Orbitals - Molecular Orbitals for Heteronuclear ... Molecular Orbitals for Heteronuclear Molecules. The molecular orbitals which describe the motion of a single electron in a molecule containing two unequal nuclear charges will not exhibit the g and u symmetry properties of the homonuclear diatomic case. The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other.
Lih molecular orbital diagram. PDF Molecular orbital DiagraM - Magadh University Molecular orbitals: Orbitals that span two or more atoms. These are constructed by overlapping atomic orbitals (AOs) which match in symmetry and size. In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! Solved QUESTION 32 Part B_Question : Draw a | Chegg.com QUESTION 32 Part B_Question : Draw a semi-quantitative molecular orbital diagram for LiH. Label the LUMO and HOMO. Attach File Browse My Computer Browse Content Collection QUESTION 33 Part B_Question : Sketch the following complexes. If more than one enantiomer exists, sketch just one. LiH - MO Diagram - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... A Molecular Orbital Energy Level Diagram of LiH | Request PDF It is considered that the usual explanations for the molecular orbitals of H-2(+), LiH, HF, and hybridization in the conventional textbooks are liable to misunderstanding. View.
A Molecular Orbital Energy Level Diagram of LiH We show an molecular orbital energy level diagram of LiH obtained by ab initioHartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. The 2σlevel of LiH is drawn at a higher position than the 1s of H in this diagram. The 1s electron of H is thus destabilized in LiH. bond - The molecular orbitals of lithium hydride - Chemistry ... Sep 19, 2018 · The occupied atomic orbitals of lithium are 1s and 2s, with energies of around -66 eV and − 5.3 e V respectively. The occupied atomic orbital of hydrogen is 1s, with an energy of -13.6 eV. Molecular Orbital Diagram Of Lih - schematron.org Jul 05, 2019 · Molecular Orbital Diagram Of Lih. We shall consider the molecular orbitals in LiH, CH and HF to illustrate how molecular orbital theory describes the bonding in heteronuclear molecules, and to. and 2p orbitals, but that is not how sodium chloride is made. Sodium atoms are Construct an MO diagram for LiH and suggest what type of bond it might have. Split orbitals for the LiH molecule | SpringerLink The four-electron problem in LiH has been treated by use of Slater type orbitals for the 1s electrons on the Li atom and split molecular orbitals for the two valence electrons. Some properties of the two dimensional spin space present in the case of four different space-functions are discussed. Total electronic energies and electric dipole moments have been calculated.
Molecular Orbitals Calculation on LiH with Algebraic ... Molecular Orbitals Calculation on LiH with Algebraic Treatment of the Integrals. In this work we employ an algebraic computational method to solve the integrals which arise in the study of diatomic molecules. Using the Slater-type orbitals (STO), we obtain analytical solutions for the one-center and two-center hybrid and Coulomb integrals. Hybridization - TU Braunschweig Hybrization. Now we want to consider the example of Lithiumhydride LiH and apply the LCAO description of molecular orbitals. Ab initio calculations (derived from the self consistent field approach) with a minimal basis (i.e. the Li atom with 1s-, 2s- and 2p-atomic orbitals and Φ 1s, Φ 2s and Φ 2p as wave function and a hydrogen wave function Φ H) produce (neglecting a minor involvement of ... PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. A Revisit to Molecular Orbitals in H2+, LiH, HF, and ... We have drawn three-dimensional contour plots of H2+ molecular orbitals and carbon hybrid orbitals according to explanations given in quantum chemistry textbooks and have also performed ab initio molecular orbital calculations of LiH and HF. Some contour representations and molecular orbital energy-level diagrams thus obtained are not consistent with the figures adopted in the textbooks. It is ...
Draw a semi-quantitative molecular orbital diagram for LiH. Draw a semi-quantitative molecular orbital diagram for LiH. Subject: Chemistry Price: Bought 3 Share With. Draw a semi-quantitative molecular orbital diagram for LiH. Label the LUMO and HOMO.
Mono- and bichromatic electron dynamics: LiH, a test case Application of the electric field induces charge movement inside the molecule and electronic transitions between the molecular orbitals. The test system is the neutral LiH molecule. The method is applied to wave functions calculated using the B3LYP (hybrid) density functional, with the STO-3G and the 6-31+G basis sets.
PDF Using Symmetry to Generate Molecular Orbital Diagrams atomic orbitals a 1g (σ g) 2s e 1u (π u) p y p x p z a 1u (σ u) atomic orbitals of terminal atoms H 1 + H 2 LGO (1) LGO (2) a 1u (σ u) …..thus, the symmetry of LGO(2) matches that of the X atom's p z - orbital …..and LGO(2) will combine with the X p z -orbital to form a new molecular orbital (MO) …..this interaction is symmetry ...
(Get Answer) - Draw a molecular-orbital diagram for LiH ... Draw a molecular-orbital diagram for LiH and write the electronic configuration. Indicate the relative energies of the Li 2s and H 1s atomic orbitals by reference to the VOIE values in Table 4-4. The first ionization energy of LiH has not been measured, but calculation predicts it to be about 8 eV.
Electronic Structure of LiH Ion (Journal Article) | OSTI.GOV Abstract. A valence-bond calculation has been carried out for (LiH) + with all twenty structures possible from Slater orbitals 1s, 2s, 2p on lithium, and 1s(= 1h) orbital on hydrogen. The total electronic energy computed for the ion at the equilibrium distance for the LiH molecule is --209.50 ev with only the 1s 2 1h structure making a significant contribution to the ground state.
Molecular Orbitals - Chem1 The lithium 1 s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
Molecular Orbital Diagram Of Lih Feb 28, 2019 · Molecular Orbital Diagram Of Lih Siagram MO diagram for dihelium looks very similar to that of dihydrogen, but each helium has two electrons in its 1s atomic orbital rather than one for hydrogen, so there are now four electrons to place in the newly formed molecular orbitals. Wiring Diagrams Free DOWNLOAD Molecular Orbital Diagram Of Lih
Molecular Orbitals of Simple Diatomic Molecules The bonding molecular orbital, H 1 (1s) + H 2 (1s), is lower in energy than the atomic orbitals. It puts most electron density between the positive nuclei for electrostatic stabilization. The antibonding molecular orbital, H 1 (1s) - H 2 (1s), has much less electron density between the nuclei and a nodal plane (a plane with zero electron density) bisecting the bond axis.
Which software to use to output molecular orbital diagrams ... I want to output a molecular orbital diagram. Can anyone recommend a software to do this? Also I searched for a python module, but didn't found a pure solution. Maybe I am using the wrong keywords. I want to connect two atoms with a specific electron configuration, to see at which energy levels they have pi- and sigma-bindings.
PDF Valence-only correlation in LiH and BeH+ - NIST coefficients are given in table 2. The first two orbitals are exactly the undisturbed Isu H. F. molecular orbital and the bonding PNO which is very similar to the second occupied H.F. molecular orbital. This orbital has been referred to as a ISH _ orbital because of the ionicity of the LiH bond [8]. However, the 2sLi and
PDF The Electronic Structure of Molecules - An Introduction to ... the direction of overlap of the p orbitals on the two atoms, Figure 2. Sigma bonds are usually stronger because the atomic p-orbitals point towards each other and have better overlap. For double bonds, the first bond is sigma type and the second bond is pi-type. When two atomic orbitals overlap, two molecular orbitals are formed. One of the
Molecular Orbitals - Molecular Orbitals for Heteronuclear ... Molecular Orbitals for Heteronuclear Molecules. The molecular orbitals which describe the motion of a single electron in a molecule containing two unequal nuclear charges will not exhibit the g and u symmetry properties of the homonuclear diatomic case. The molecular orbitals in the heteronuclear case will in general be concentrated more around one nucleus than the other.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
PDF A Molecular Orbital Study of the Dipole Moment of HF, LiH ... In the ab initio molecular orbital calculations, the widely used 6-311G** basis sets are adopted throughout this study. 7,8 We use the MCSCF wave functions in order to treat the dissociation limit correctly. We use CASSCF within the configuration of 2 electrons in 2 orbitals for LiH, full-CI for

0 Response to "40 lih molecular orbital diagram"
Post a Comment