41 match the diagram below to the transition metal it represents.
Match The Diagram Below To The Transition Metal It Represents ... Match the Diagram Below to the Transition Metal It Represents. band theory match the diagram to the transition metal it match the diagram to the transition metal it represents what band represents the truest form of metal music which albums by metal mastering chemistry chapter 2 assignment flashcards match each diagram to the atom or ion it represents drag each item to the appropriate bin 10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
Chem Exam #2 (Chem 101 Ch 3 hw) Flashcards - Quizlet Start studying Chem Exam #2 (Chem 101 Ch 3 hw). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
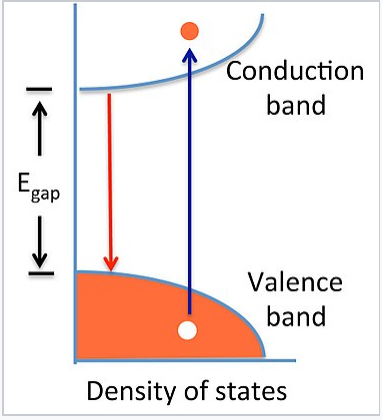
Match the diagram below to the transition metal it represents.
Solved Band theory is an extension of molecular ... - Chegg Transition metals from; Question: Band theory is an extension of molecular orbital theory According to band theory, atomic orbitals between atoms in a sample form a nearly continuous band of molecular orbitals. In a metal, the bottom half of the band is composed of bonding molecular orbitals and the top half of the band is composed of anti ... Biochem Exam 2 Flashcards - Quizlet Z represents O2 binding in cells with elevated 2,3-BPG, whereas X represents O2 binding in cells with decreased 2,3-BPG In one type of hemoglobin mutant the amino acid change generates a strong ionic interaction stabilizing the T state conformation but only under conditions of lower pH, e.g., at pH 7.2 compared to pH 7.6. Which of the following transitions would result in the ... We see from the energy level diagram that the energy levels get closer together as #n# increases. This, the smallest energy and the longest wavelength is associated with the #n = 7 → n = 8# transition. I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead.
Match the diagram below to the transition metal it represents.. Chem ch 3 smartworks hw: Flashcards - Quizlet Match the six colors with the appropriate part of the hydrogen energy-level diagram to indicate the color of each emission. F (starting at n = infinity) to C to D to A to E to B (ends at n =1). Using only the periodic table, arrange each set of particles by relative radius. Reaction Coordinate Diagrams - University of Illinois ... The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. ... The transition state is the point of highest energy between the reactant(s) and product(s). ... such as the platinum metal added to the H 2 /O 2 mixture ... Transition Metals Webinar Extra Questions & ANSWERS This diagram represents the energy change that occurs when a d electron in a transition metal ion is excited by visible light. (a) Give the equation that relates the energy change ΔE to the Planck constant h and the frequency of the visible light v. chem drop test (solutions, acids and bases, kinetic ... - Quizlet The diagrams below represent an ionic crystal being dissolved in water. According to the diagrams, the dissolving process takes place by ... (aq) transition metal ...
Stress-strain Diagram | Strength of Materials Review at ... The diagram shown below is that for a medium-carbon structural steel. Metallic engineering materials are classified as either ductile or brittle materials. A ductile material is one having relatively large tensile strains up to the point of rupture like structural steel and aluminum, whereas brittle materials has a relatively small strain up to ... Che 140 Ch 7 Learn Smart Flashcards - Quizlet Match each type of metal with the correct statement. ... Match each equation to the process involved for the ionization energy that it represents (M=metal) ... Use the orbital diagram to list the following sublevels in order of decreasing energy. The highest-energy sublevel belongs at the top of the list. Coordination Complexes and Ligands - Purdue University Coordination Complexes. Coordination compounds, such as the FeCl 4-ion and CrCl 3 6 NH 3, are called such because they contain ions or molecules linked, or coordinated, to a transition metal.They are also known as complex ions or coordination complexes because they are Lewis acid-base complexes. The ions or molecules that bind to transition-metal ions to form these complexes are called ligands ... Mastering Chemistry: Chapter 2 Assignment Flashcards - Quizlet Mastering Chemistry: Chapter 2 Assignment. What is the ratio of hydrogen atoms (H) to oxygen atoms (O) in 2 L of water? Enter the simplest whole number ratio in order of hydrogen to oxygen, respectively. Express your answer as two integers, separated by a comma (e.g., 3,4).
PDF ANSWER. Series #2 - LSU The transition labeled "e". 4. Of the five separate electron transitions that have been labeled with letters in the energy-level diagram, which results in the production (or destruction) of the longest wavelength photon? ANSWER. The transition labeled "c". 5. Given that the "Hα" absorption line identified in the hydrogen spectrum ... 6.4 Electronic Structure of Atoms (Electron Configurations ... The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals that are shown in Figure 3 or Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among others, are not those we would ... Solved Band theory is an extension of molecular orbital ... Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents. Chapter 5 Concept Quiz Flashcards | Quizlet B. You can tell because the electron jumps up from 0 eV to 10.2 eV. The diagram represents energy levels in a hydrogen atom. The labeled transitions (A through E) represent an electron moving between energy levels. Suppose that an electron in a hydrogen atom absorbs 10.2 eV of energy, so that it moves from level 1 to level 2.
Energy Level and Transition of Electrons | Brilliant Math ... Hence in the figure above, the red line indicates the transition from n = 3 n=3 n = 3 to n = 2, n=2, n = 2, which is the transition with the lowest energy within the Balmer series. Recall that the energy level of the electron of an atom other than hydrogen was given by E n = − 1312 n 2 ⋅ Z eff 2 kJ/mol .
Answer Key: ATOM and PT Study Guide and ... - Google Search The chart below shows the percentages of elements in the Earth's crust. Excluding the "Other" category, what percentage of the Earth's crust is . a. alkali metals? 5.4%. b. alkaline-earth metals? 5.6%. INTERPRETING GRAPHICS . 19. Study the diagram below to determine the pattern of the images. Predict the missing image, and draw it ...
Match the diagram below to the transition metal it represents. The diagram below represents currents in a segment of an electric circuit For the following question, match the key event of meiosis with the stages listed below. Which of the following is true about the metal boxes pictured below
PDF Name: Period: IPS Unit 8 Periodic Table Review Worksheet 11. An electron dot diagram uses the symbol of an element and dots to represent the (quarks/electrons) in the outer energy level. Atoms 1. have a mass number equal to are organized the sum of as elements in the are made up of 4. 5. 2. 6. and and and
Part A Match each diagram to the atom or ion it represents ... Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. You did not open hints for this part. ANSWER: Part B What is the charge on the most stable ion of each of the following elements? Drag each item to the appropriate bin. You did not open hints for this part.
2.5 The Periodic Table - Chemistry The elements can also be classified into the main-group elements (or representative elements) in the columns labeled 1, 2, and 13-18; the transition metals in the columns labeled 3-12; and inner transition metals in the two rows at the bottom of the table (the top-row elements are called lanthanides and the bottom-row elements are actinides ...
PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... In octahedral complexes, the molecular orbitals created by the coordination of metal center can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. The metal orbitals taking part in this type of bonding are nd, (n+1)p and (n+1)s. It should be noted down
PDF Chapter 24 Chemistry of Coordination Compounds • suggested in 1893 that metal ions have primary and secondary valences. ! Primary valence equals the metal's oxidation number ! Secondary valence is the number of atoms directly bonded to the metal (coordination number) Co(III) oxidation state Coordination # is 6 Cl-
Which of the following transitions would result in the ... We see from the energy level diagram that the energy levels get closer together as #n# increases. This, the smallest energy and the longest wavelength is associated with the #n = 7 → n = 8# transition. I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead.
Biochem Exam 2 Flashcards - Quizlet Z represents O2 binding in cells with elevated 2,3-BPG, whereas X represents O2 binding in cells with decreased 2,3-BPG In one type of hemoglobin mutant the amino acid change generates a strong ionic interaction stabilizing the T state conformation but only under conditions of lower pH, e.g., at pH 7.2 compared to pH 7.6.
Solved Band theory is an extension of molecular ... - Chegg Transition metals from; Question: Band theory is an extension of molecular orbital theory According to band theory, atomic orbitals between atoms in a sample form a nearly continuous band of molecular orbitals. In a metal, the bottom half of the band is composed of bonding molecular orbitals and the top half of the band is composed of anti ...

0 Response to "41 match the diagram below to the transition metal it represents."
Post a Comment