42 gold foil experiment diagram
Rutherford's Gold Foil Experiment Rutherford's Gold Foil Experiment. History -. Before Rutherford, the most widely accepted theory about the distribution of charges and masses on How did the plum pudding fail for later research? Ernest Rutherford did similar experiments in which he sent alpha beams or alpha particles over a... Gold Foil Experiment — Overview & Importance - Expii The Gold Foil Experiment was conducted to prove the existence of the positively charged atomic nucleus and the "orbital" model, where electrons orbit Gold Foil - The importance of gold foil was that Rutherford and Geiger could use a very thin strip of material for the alpha particles to interact with.
history - Alpha particle deflection by 180 degree in Rutherford's gold... Rutherford's experiment looked much like this As you can see, the incoming alpha particles hit the gold foil and could scatter in multiple directions, but the detector went around the whole foil (sparing some small region so that the alpha particles could enter the experiment) so even back scattered...
Gold foil experiment diagram
Rutherford's Gold Foil Experiment ~ ChemistryGod Rutherford's Gold Foil Experiment, Rutherford's Alpha Particles Scattering Experiments, Geiger-Marsden Experiments. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science. What is the Rutherford gold-foil experiment? | Britannica The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford's model to make his own. In Bohr's model the orbits of the electrons were explained... The Gold Foil Experiment Describe Rutherford's gold foil experiment. Describe the nuclear model of the atom. How much space do bricks occupy? The Gold Foil Experiment. In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the...
Gold foil experiment diagram. Rutherford and the Gold Foil Experiment Ernest Rutherford's Gold Foil Experiment. Day 1: Introductory video about the development of the current atomic theory. Day 2 and 3: Students will Day 4: Students will read the three printed articles that present Rutherford's experiment in detail. The students will answer the following questions to... Rutherford's Gold Foil Experiment by Aly Young • Gold Foil (Au) - Gold foil is composed of gold particles, and therefore has an overall positive charge of either 1+ or 3+. So what most people are wondering is so how did Rutherfords gold foil experiment contribute to the understanding of the structure of an atom and a tiny dense nucleus? Rutherford's Gold Foil Experiment: Setup, Analysis and Conclusion Rutherford's Gold Foil Experiment was an important experiment which revealed a lot about the structure of an atom and changed the world's perspective of The Atomic model proposed by Ernest Rutherford was the 'Planetary Model' and was devised on the basis of the Gold Foil Experiment. Chemistry / Rutherford's Gold Foil Experiment Having this diagram open as you read may help you understand the experiment. Please note: an alpha particle is a helium atom that has had its electrons removed. When Rutherford's assistants bombarded very thin foils of gold with alpha particles, they observed something quite different.
Significance of Rutherfords Gold Foil Experiment | Actforlibraries.org Gold foil can be used for a lot of things, especially if you are a creative person, and I am. However, one thing I never thought about doing with gold foil was to use it to conduct a scientific experiment. Ernest Rutherford, though, used it to do just that. His experiment produced amazing results (and not just... Rutherford's Atomic Model - Gold Foil Experiment, Results... Gold Foil was bombarded with positively charged α-particles. He used α-particles because they are very energetic and are heavier than other particles. Rutherford came out with a different hypothesis after the experiment and the results ruled out the Plum Pudding structure of an Atom. What is the 'Gold Foil Experiment'? | Live Science The gold foil experiments gave physicists their first view of the structure of the atomic nucleus and the physics underlying the everyday world. Here, an illustration of Rutherford's particle scattering device used in his gold foil experiment. (Image credit: BSIP/UIG Via Getty Images). Geiger-Marsden Experiments - Rutherford gold foil experiment Geiger-Marsden experiments (also called the Rutherford gold foil experiment) were performed between 1908 and 1913 by Hans Geiger (of Geiger Rutherford's new model for the atom is based on the experimental results, which were obtained from Geiger-Marsden experiments (also called the...
New Page 2 | The Gold Foil Experiment (Ernest Rutherford) Gamma-rays are not affected by an electric field and therefore have no electric charge. The Gold Foil Experiment (Ernest Rutherford). Rutherford began his graduate work by studying the effect of x-rays on various materials. Shortly after the discovery of radioactivity, he turned to the study of the... Gold foil experiment - Academic Kids The gold foil experiment was an experiment done by Ernest Rutherford to determine the layout of the atom. Until that time, the prevailing theory was the He determined this by bombarding gold foil with alpha particles, and observing the scattering of these particles, a procedure requiring many hours in a... Diagram of chem gold foil experiment diagram | Quizlet gold foil. ... beam of alpha particles. ... sulfide screen. chem cathode ray tube diagram. Size of The Nucleus - Rutherford Gold Foil Experiment Rutherford Gold Foil Experiment. J.J Thompson in 1898, proposed a model of the atom which looked more or less like plum pudding are raisin pudding. He assumed an atom to be a spherical body in which electrons are unevenly distributed in a sphere having a positive charge which balances the electron's...
Rutherford's gold foil experiment (video) | Khan Academy Learn about Rutherford's discovery of the nucleus and the development of the nuclear model of the atom.
Gold Foil Experiment ( Read ) | Chemistry | CK-12 Foundation Gold Foil Experiment. Rutherford's discovery of the nucleus and the planetary model of the atom. ShowHide Details. Description. Discusses Rutherford's gold foil experiment and the nuclear model of atom.
The Gold foil experiment - PDF Free Download 2 cgrahamphysics.com 2016 The Gold foil experiment & history Learning Outcomes 1. Investigate the history of the discovery of the atom as we know it Why were alpha particles used and not electrons? Include your findings with a diagram on your poster. The Gold foil experiment Task 4: Explain the...
Simulation Rutherford`s Gold Foil Experiment Behind the gold foil was a screen that would light up when a particle hit it. He noticed that most of the particles went straight through the foil and only a You will simulate this experiment with marbles. By rolling a marble at 5 targets and counting how many times they hit, you will be able to calculate the...
About Rutherford's Gold Foil Experiment | Sciencing The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester...
Geiger-Marsden experiments - Wikipedia The Geiger-Marsden experiments (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated.
Rutherford's Gold Foil Experiment - Chemistry | Socratic Rutherford's diffraction experiment tests diffraction via a thin foil made of gold metal. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. The passing of many of the particles through suggested the condensed nucleus version of the atom model.
Rutherford's Gold Foil Experiment The gold foil experiment was a pathbreaking work conducted by scientists Hans Geiger and Ernest Marsden under the supervision of Nobel laureate physicist Ernest Rutherford that led to the discovery of the proper structure of an atom. Known as the Geiger-Marsden experiment, it was performed at the...
Christina's Chemistry Blog: Rutherford's Gold Foil Experiment Rutherford's gold foil experiment proved the "plum pudding model" of the atom incorrect, which allowed for a more complete understanding of the atom. His discovery of the nucleus and atomic structure was refined by Niels Bohr. Niels Bohr designed a model of the atom based off of...
Ernest Rutherford's Gold Foil Experiment - Atomic Structure - YouTube Walkthrough of Ernest Rutherford's gold foil experiment--an experiment that dramatically changed the way scientists think about the structure of an atom....
Discovering the Nucleus: Rutherford's Gold Foil Experiment | ChemTalk The gold-foil experiment disproved J.J. Thomsons plum pudding model, which hypothesized the atom was positively charged spaced with electrons embedded inside. Therefore, giving way to the nuclear model. In this model, Rutherford theorized the atomic structure was similar to that of the solar system.
Rutherford gold foil experiment Rutherford gold foil experiment. 1. ASSIGNMENT CHEMISTR Y By: Rohit Kumar. 2. RUTHERFORD'S SCATTERING EXPERIMENT Rutherford in 1911, performed some scattering experiments in which he bombarded thin foil of gold with a beam of fast moving alpha particles.
The Gold Foil Experiment Describe Rutherford's gold foil experiment. Describe the nuclear model of the atom. How much space do bricks occupy? The Gold Foil Experiment. In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the...
What is the Rutherford gold-foil experiment? | Britannica The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford's model to make his own. In Bohr's model the orbits of the electrons were explained...
Rutherford's Gold Foil Experiment ~ ChemistryGod Rutherford's Gold Foil Experiment, Rutherford's Alpha Particles Scattering Experiments, Geiger-Marsden Experiments. The Rutherford gold foil experiment or alpha particles scattering experiment remains a famous experiment in the history of science.
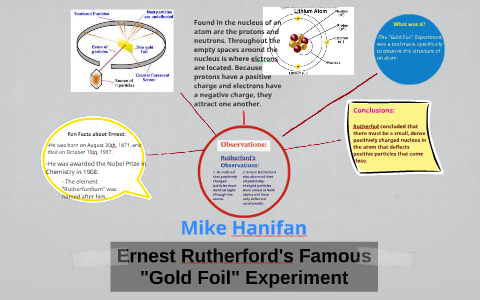

0 Response to "42 gold foil experiment diagram"
Post a Comment