42 molecular orbital diagram for cn
Molecular Orbital Diagrams simplified | by Megan... | Medium Molecular Orbital Diagrams simplified. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the...
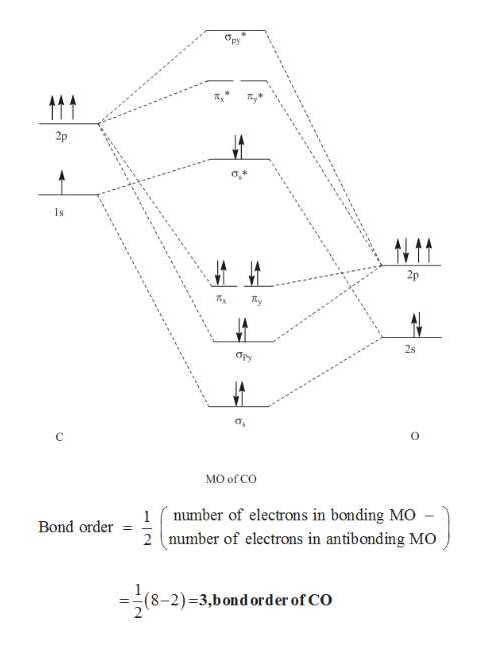
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Accordingly, a molecular orbital diagram such as Figure 9-5 is inappropriate for heteronuclear diatomic molecules. If the two elements are similar (as in NO or CN mole-cules, for example), we can modify the diagram of Figure 9-5 by skewing it slightly. Figure 9-7 shows the energy level diagram...
Molecular orbital diagram for cn
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the help of Valence Bond Theory. Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. in molecules. Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore.
Molecular orbital diagram for cn. Molecular Orbitals - an overview | ScienceDirect Topics Figure 5. Molecular orbital diagram for a simple octahedral complex. This is qualitatively equivalent to the results of the simpler crystal field theory, which ignores orbital overlap with the ligand orbitals and uses just the electrostatic repulsion of the metal d-orbitals and the negative charges of the ligands. PDF Figure 9.32: The molecular orbital energy-level diagram for • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the... PDF lecture_6 Molecular orbital 'resembles' the atomic orbital to which it lies closest in energy. Always break MO diagrams down into components based on symmetry. Walsh diagrams summarise changes in MO diagram wrt structure note a combination of first and second order effects. Molecular Orbital Diagram For Cn - Wiring Diagram Source Complete this molecular orbital diagram for cn then determine the bond order. Vijayta gupta is right the n atom is lower in energy.
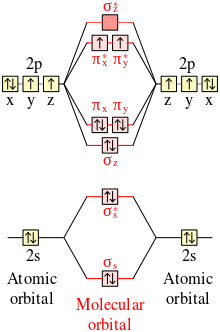
Molecular Orbital Diagrams of Diatomic Molecules - Chem Lewis Structure: Molecular Orbital Energy Diagram. Total # of bonding electrons. The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can... PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. Lecture 4 Revision of hybridisation Molecular orbital theory and diatomic molecules. BeH2 BF3, CO32-C2H4 SO42-, CH4, NH3, H2O, PCl5, SF4. SF6 [Ni(CN)4]2-[PtCl4]2-. 66. Asked for: "skewed" molecular orbital energy-level diagram, bonding... Unlike earlier diagrams, only the molecular orbital energy levels for the molecules are shown here. For simplicity, the atomic orbital energy levels for Calculate the total number of valence electrons in CN − . Then place these electrons in a molecular orbital energy-level diagram like Figure 4.10.4 in... 8.4 Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O2. We draw a molecular orbital energy diagram similar to that shown in Figure 11. Each oxygen atom contributes six electrons, so the diagram appears as...
Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable. Molecular orbital diagram — Wikipedia Republished // WIKI 2 Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Molecular Orbital diagram of CN- - YouTube Molecular Orbital diagram of CN-. Смотреть позже. Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
Molecular Orbital Theory: Energy level diagram for... Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular...
Solved Complete this molecular orbital diagram for CN... | Chegg.com Transcribed image text : Complete this molecular orbital diagram for CN then determine the bond order. Note that the 1s orbital is not shown in this problem. To add arrows to the MO diagram, click on the blue boxes. Bond order of CN O 0 O 0.5 Molecular orbitals of CN Atomic orbitals of Atomic...
How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy to both create and Dot diagrams are very different to orbital diagrams, but they're still very easy to understand.
Asked for: molecular orbital energy-level diagram, valence... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we...
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Energy level diagram for Molecular orbitals - Chemical... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. We draw a molecular orbital energy diagram similar to that shown in Figure 8.37. Each oxygen atom contributes six electrons, so the diagram...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Molecular orbital diagram - Infogalactic: the planetary knowledge core A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
What is the molecular orbital energy diagram of CO? - Quora A molecular orbital diagram , or MO diagram , is a qualitative descriptive tool explaining chemical bonding. in molecules. Here is the molecular orbital diagram of CN-: There are 8 bonding electrons and 2 antibonding electrons, therefore.
Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: It is used to define the bonding in molecules which cannot be explained with the help of Valence Bond Theory. Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals.
MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available

0 Response to "42 molecular orbital diagram for cn"
Post a Comment