39 orbital diagram for cl
techiescientist.com › pcl3-lewis-structurePCl3 Lewis Structure, Hybridization ... - Techiescientist Apr 21, 2022 · In a molecular orbital diagram of PCl3, we can see 3 bonding orbitals, which will be occupied. And 3 anti-bonding orbitals which will be empty. We can see from the hybridization that 3 Sp3 hybrid orbitals of phosphorus will be occupied by 3 Cl atoms. The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of ... PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4.
What is the molecular orbital diagram for HCL? - Quora Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the H electron and therefore are allowed to mix.
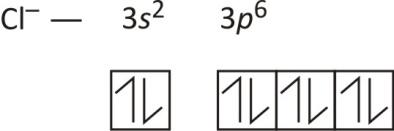
Orbital diagram for cl
Electron configuration for Chlorine (element 17). Orbital ... Chlorine electron configuration. ← Electronic configurations of elements. Electronic configuration of the Chlorine atom: 1s 2 2s 2 2p 6 3s 2 3p 5. Reduced electronic configuration Cl: [Ne] 3s 2 3p 5. Below is the electronic diagram of the Chlorine atom Distribution of electrons over energy levels in the Cl atom. 1-st level (K): 2. Electron Configuration for Chlorine (Cl) - UMD In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Cl Atomic Orbitals - uwosh.edu The green dot represents the location of a chlorine nucleus (significantly enlarged so that you can see it). On the right hand side are four pull-down menus from which you can choose an orbital to display. The electronic configuration of a ground state chlorine is [Ne]3s 2 3p 5 (1s 2 2s 2 2p 6 3s 2 3p 5 ).
Orbital diagram for cl. topblogtenz.com › cl2-lewis-structure-molecularCl2 lewis structure, Molecular shape, Polar or Non-Polar, Dot ... The hybridization of each chlorine atom in the Cl2 lewis structure is Sp³. It means it has one s orbital and three p orbital. And this makes four hybrid orbitals. In order to find the hybridization of the chlorine atom in the Cl2 molecule, we have to find its steric number. ⇒ Steric number = Number of atoms attached + Number of lone pairs on ... Molecular Orbital Diagram For Cl2 - schematron.org Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. Solved Create the atomic orbital diagram for chlorine. In ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (15 ratings) Transcribed image text: Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons? How can I draw an orbital diagram for chloride ion class ... The orbital diagram of valence shell of chlorine is $\text{Cl}-3{{s}^{2}}3{{p}^{5}}$ In an orbital, electrons are filled according to Hund's Rule, Autobuses rule and Paul's exclusion principle. Here if chlorine atoms gain electrons then it forms chloride ions.
Cl2 Molecular Orbital Diagram Cl2 Molecular Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Molecular Orbital Diagram For Cl2 Cl2 molecular orbital diagram. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. Energy of. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of chlorine molecule is 1. How do I determine the bond order using the Lewis structure?. Draw the orbital diagram of CH3Cl. - Sarthaks eConnect ... Draw the orbital diagram of CH 3 Cl. class-12; Share It On Facebook Twitter Email. 1 Answer. 0 votes . answered Apr 26, 2019 by Anishk (59.1k points) selected Apr 27, 2019 by Vikash Kumar . Best answer. Diagram of CH 3 Cl. ← Prev Question ... How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Chlorine Orbital diagram, Electron configuration, and ... Orbital diagram for Chlorine The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom, it uses an arrow to represent the electrons, every orbital (one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1). Complete the atomic orbital diagram for the ground-state ... Complete the atomic orbital diagram for the ground-state electronic configuration of chlorine. Atomic Orbitals: An Atomic Orbital represents the place where there is a maximum probability of... your-online.ru › en › electronic-formulasElectron configuration for Molybdenum (element 42). Orbital ... The order of filling the orbitals with electrons in the Mo atom is an exception to the rule. Expected electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 4 But in reality, one electron moves from the 5s orbital to the 4d orbital: terpconnect.umd.edu › ~wbreslyn › chemistryElectron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Molecular orbital diagrams of Cl2, H2O, and Br2 ... Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
socratic.org › questions › what-kind-ofWhat kind of intermolecular forces are present in the ... Aug 16, 2016 · "CCl"_4 is a tetrahedral molecule with a "Cl-C-Cl" bond angle of 109.5°. The two "C-Cl" bond dipoles in the plane of the paper have a resultant pointing to the right at an angle of 54.75° from the vertical. The two "C-Cl" bond dipoles behind and in front of the paper have an equal and opposite resultant to the first.
ocov2.jpl.nasa.govOrbiting Carbon Observatory-2: Home Site Manager: Karen Yuen Webmaster: David Martin JPL Clearance: CL#08-3636. top. Back to top ...
What is the electron configuration of Cl-? | Socratic The chloride ion, Cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. The overall number of electrons is now 18. Thus, the electron configuration for Cl− should be. 1s2 2s2 2p6 3s2 3p6 = 18 electrons. Answer link.
opentextbc.ca › 8-4-molecular-orbital-theory8.4 Molecular Orbital Theory – Chemistry The region of space in which a valence electron in a molecule is likely to be found is called a molecular orbital (Ψ 2). Like an atomic orbital, a molecular orbital is full when it contains two electrons with opposite spin. We will consider the molecular orbitals in molecules composed of two identical atoms (H 2 or Cl 2, for example).
Sodium(Na) electron configuration and orbital diagram These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f. Determining the value of 'l' for different energy levels is- If n = 1, (n - 1) = (1-1) = 0 Therefore, the orbital number of 'l' is 1; And the orbital is 1s. If n = 2,
How to Write the Orbital Diagram for Chlorine (Cl) - YouTube To write the orbital diagram for the Chlorine atom (Cl) first we need to write the electron configuration for just Cl. To do that we need to find the number...
Answered: 15. The orbital diagram for the valence… | bartleby The orbital diagram for the valence shell of Cl contains 8 full orbitals and 1 half-filled orbital a) b) 6 paired electrons and 1 unpaired electron 8 paired electrons 2 full orbitals and 1 half-filled orbital c) d) Expert Solution. Want to see the full answer? Check out a sample Q&A here.
Draw the Orbital Overlap Diagram for HCl (Hydrochloric ... Chlorine is sp3-hybridized, and so there are four tetrahedrally-arranged 3sp3 orbitals surrounding it.Hydrogen does not hybridize, so it's simply a circle fo...
ClO2 molecular orbital diagram? - Chemistry Stack Exchange ClO2 is of C2v point group, so I just read off the C2v character table and got the following group orbitals [where z axis is that of the principal axis, and the outer atoms are aligned such that the y-axis points towards the centre atom]: Adding those to the s orbital (A1), px orbital (B2), py orbital (B1) and pz orbital (A1) of the chlorine ...
How to Draw Orbital Diagrams for any atom | Orbital Notation The orbital diagram is drawn by using three rules - Aufbau's rule, Hund's rule, and Pauli's exclusion rule. For drawing the orbital diagram or orbital notation, first, find the number of electrons in an atom then write its electron configuration to determine which orbital should be filled. And then fill the electrons in empty orbital ...
What is orbital diagram for chlorine? - Answers What is an orbital diagram? An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to...
Chlorine(Cl) electron configuration and orbital diagram Orbital diagram for chlorine (Cl) Chlorine (Cl) excited state electron configuration Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The valency of the element is determined by electron configuration in the excited state.
Give the orbital diagram for an atom of Cl. | Study.com Give the orbital diagram for an atom of {eq}Cl {/eq}. Electron Configuration: We use three rules to help determine how to draw the orbital diagrams (and thus get the electron configuration) for atoms:
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
PDF Orbital diagram of chlorine 17 - Weebly Orbital diagrams are pictorial representations of the electronic configuration, showing the individual orbitals and the electron pairing arrangement. We start with a single hydrogen atom (atomic number 1), which consists of a proton and an electron. Referring to Figure 3 or Figure 4, we would expect to find the electron in orbital 1s.
Cl Atomic Orbitals - uwosh.edu The green dot represents the location of a chlorine nucleus (significantly enlarged so that you can see it). On the right hand side are four pull-down menus from which you can choose an orbital to display. The electronic configuration of a ground state chlorine is [Ne]3s 2 3p 5 (1s 2 2s 2 2p 6 3s 2 3p 5 ).
Electron Configuration for Chlorine (Cl) - UMD In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
Electron configuration for Chlorine (element 17). Orbital ... Chlorine electron configuration. ← Electronic configurations of elements. Electronic configuration of the Chlorine atom: 1s 2 2s 2 2p 6 3s 2 3p 5. Reduced electronic configuration Cl: [Ne] 3s 2 3p 5. Below is the electronic diagram of the Chlorine atom Distribution of electrons over energy levels in the Cl atom. 1-st level (K): 2.

0 Response to "39 orbital diagram for cl"
Post a Comment