37 build the orbital diagram for the ion most likely formed by phosphorus.
Express your answer numerically as an integer.Build the orbital diagram for the ion most likely formed by phosphorus. FREE Expert Solution. Iron (Fe) has an atomic number of 26. The ground state electron configuration of the Fe atom is: ... Build the orbital diagram for the ion most likely formed by phosphorus. Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Build the orbital diagram for the ion most likely formed by phosphorus. ... explain how you would build a ball-and-stick model of phosphorus trichloride. Include a description of the parts of the model. ... spontaneously bursts into flame in oxygen. if 6.5g of white phosphorus reacts with sufficient oxygen to form 11.54g of a phosphorus oxide ...
Build the orbital diagram for the ion most likely formed by phosphorus.
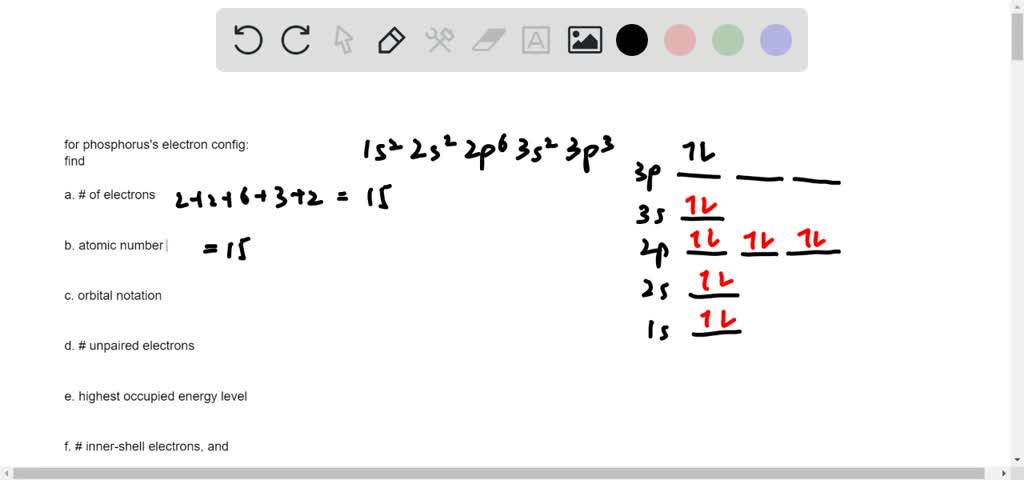
Part B:Build the orbital diagram for the ion most likely formed by phosphorus. Phosphorous forms an ion with a charge of -3, which means it has the same electron configuration as Argon. That electron configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6, These 10 chemistry test questions deal with the concepts of electronic structure and configuration. draw the orbital notation C. the subshell of p orbitals _____ c. Consider the orbital diagram: 1. Build the orbital diagram for the ion most likely formed by phosphorus. What is the electron configuration of Iron? 66. Electrons move around the atomic nucleus in specific regions, called orbitals. An orbital is a region of space that one or two electrons can occupy. Even though one or two electrons may occupy a given orbital, the most stable and balanced condition occurs when the orbital contains two electrons.
Build the orbital diagram for the ion most likely formed by phosphorus.. Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link. Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. Draw ... The atomic number of phosphorus is 15. Thus, phosphorus atom contains 15 electrons. The order of filling of energy levels is 1 s, 2 s, 2 p, 3 s, 3 p, 4 s,. 15 electrons of phosphorus atom will fill up to 3 p orbital, which will contain three electrons: last electron added is 3 p Electron. Therefore, N = 3 and, for p - type orbital, l = 1. Build the orbital diagram for the ion most likely formed by phosphorus. chemistry. Which of the following diatomic species are paramagnetic and which are diamagnetic? A blank molecular orbital diagram (Part B 1 figure) has been provided to help you. Drag the formulas to the appropriate magnetic bin . Chemistry
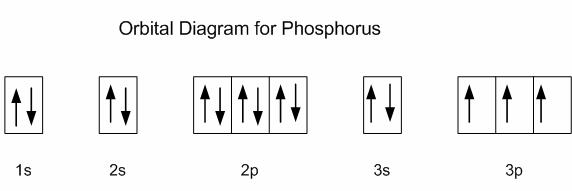
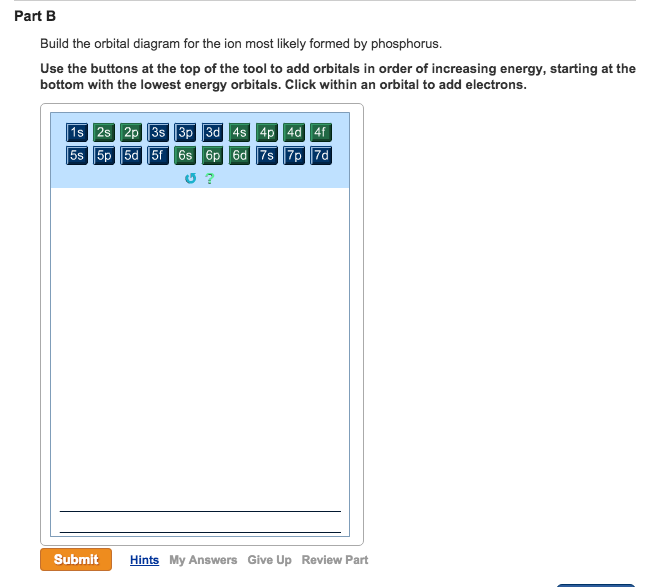
Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Build the orbital diagram for the ion most likely formed by phosphorus? 1s22s22p63s23p3 is for Phosphorus and the most likely ion is to be a 3- because it wants to have a full outer shell ... Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top or the tool to add orbitals in order of increasing energy, starting at the energy orbitals. Click within on orbital to add electrons.
What is the orbital diagram for silicon? 0 votes . 2.5k ... Since 1s can only hold two electrons the next 2 electrons for Silicon go in the 2s orbital. The nex six electrons will go in the 2p orbital. ... 1 answer. build the orbital diagram for the ion most likely formed by phosphorus. asked Aug 25, 2020 in Other by megha00 Expert (30.2k points ... Build the orbital diagram for the ion most likely formed by phosphorus. answer ch 4 hw #14: Each element in the periodic table has a distinctive atomic_____. radius: Place the following elements in order of decreasing atomic size: antimony, selenium, fluorine, rubidium, strontium, and arsenic. Rubidium, Strontium, Antimony, Arsenic, Selenium ... Construct the orbital diagram of the f ion. Solved show the orbital diagrams for following chromi. Electron Shells Orbitals The Periodic Table Article Khan Academy Build the orbital diagram for the ion most likely formed by phosphorus. Construct the orbital diagram of the f ion. This video shows you how to write the electron configuration for ... Build the orbital diagram for the ion most likely formed by phosphorus. 1s2 2s2 2p6 3s2 3p6. Phosphorous forms an ion with a charge of 3 which means it has the same electron configuration as argon. Visit the post for more. Asked by morgan on january 23 2015. That electron configuration is. Click wtihin an orbital to add electrons.

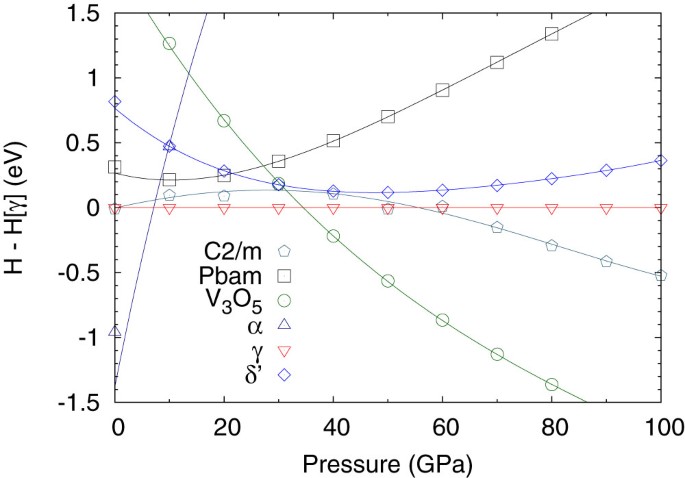
Novel Superconducting Skutterudite Type Phosphorus Nitride At High Pressure From First Principles Calculations Scientific Reports
Build The Orbital Diagram For The Ion Most Likely Formed By Phosphorus. Orbital for ion most likely formed by phosphorus? Each element in the periodic table has a distinctive The more electrons that are lost, the smaller the ion becomes. more electrons gained, larger the ion becomes.
Build the orbital diagram for the ion most likely formed by phosphorus. Does anyone know what it is and how to answer it on mastering chemistry, that **** is so confusing. for mastering chem make sure the lower numbers r at the bottum. great insightful answers, thank you.

Atomic Site Electrocatalysts For Water Splitting Oxygen Reduction And Selective Oxidation Chemical Society Reviews Rsc Publishing
Carbon dioxide was the first gas to be described as a discrete substance. In about 1640, the Flemish chemist Jan Baptist van Helmont observed that when he burned charcoal in a closed vessel, the mass of the resulting ash was much less than that of the original charcoal.
Click wtihin an orbital to add electrons. Question: Part BBuild the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click wtihin an orbital to add electrons.
Energy of atomic orbitals increases as the principal quantum number, n, increases. In any atom with two or more electrons, repulsion between electrons makes energies of subshells with different values of l differ so that energy of orbitals increases within the shell in order s . p d F. Figure 1 depicts how these two trends in increasing energy relate. 1 s orbital at the bottom of the diagram ...
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus.
Expressed as a negative logarithm of the hydrogen ion concentration in a solution, pH = -log10[H+]. If the hydrogen ion concentration of a solution increases, the pH will decrease, and vice versa. The value for pure distilled water is regarded as neutral, pH values from 0 to 7 indicate acidity, and from 7 to 14 indicate alkalinity.
Should – Has = 5 – 4 = +1 For calculating the formal charge of an atom in any compound, you need to know what is the bonding structure of the compound. Drawing Lewis Structures - Oneonta Lewis structures can show us when double and triple bonds are most likely, or perhaps the only kind of bonding that make a molecule possible.
Build the orbital diagram for the ion most likely formed by phosphorus. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add...
The 2s orbital looks much like the 1s orbital except that the electron is more likely to be found further from the nucleus. The bonds that are formed are called the Sp3 bond and the Sp2 bond.
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus, P, 1s22s22p63s23p3 (), [Ne]3s 3p, P, p-block, Gp5/ Build the orbital diagram for the ion most likely formed by phosphorus? a \ will be an arrow going one way and a / will be the other way. a [ ] represents a box. Phosphorus atomic orbital and ...
Academia.edu is a platform for academics to share research papers.
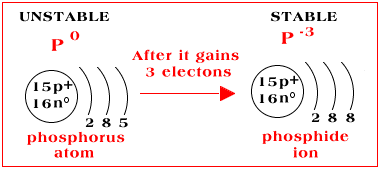
Build the orbital diagram for the ion most likely formed by phosphorus. Click within an orbital to add electrons. That electron configuration is. In chemistry a phosphide is a compound of phosphorus with a less electronegative element or elements. 16 points write the electron configuration for h.
Build the orbital diagram for the ion most likely formed by phosphorus. Phosphorous forms an ion with a charge of -3, which means it has the same electron configuration as Argon. That electron configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6-- a total of 18 electrons.

Black Phosphorus Quantum Dots In Inorganic Perovskite Thin Films For Efficient Photovoltaic Application
Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is most widely distributed in space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide (silica) or silicates.
30 Build The Orbital Diagram For The Ion Most Likely ... Part B Build the orbital diagram for the ion most likely ... 34 Build The Orbital Diagram For The Ion Most Likely ... High School Chemistry/Orbital Configurations - Wikibooks ... 8.4 Molecular Orbital Theory - Chemistry Orbital Box Diagram Phosphorus Aufbau Diagram For Phosphorus
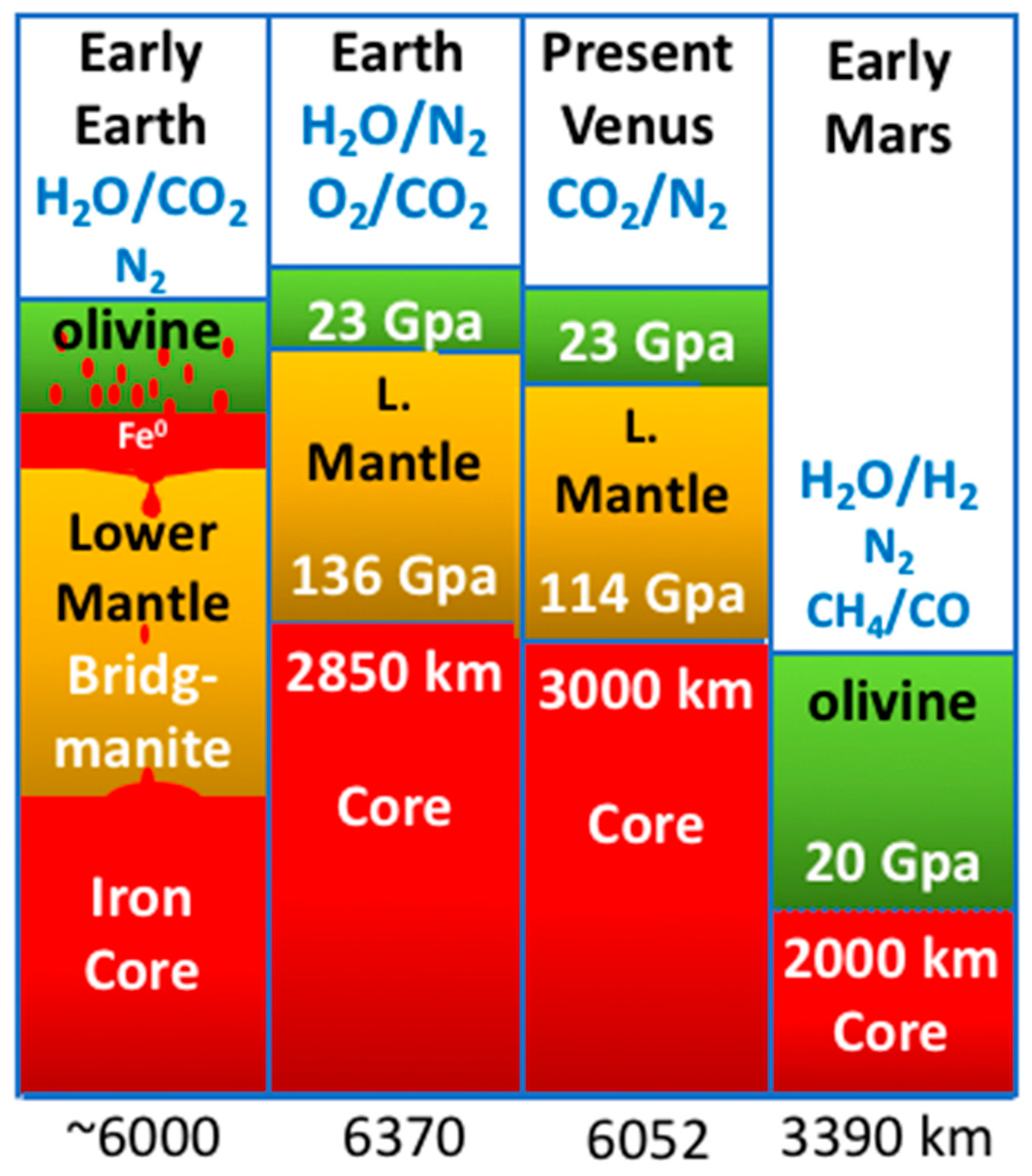
Life Free Full Text Six Must Have Minerals For Life S Emergence Olivine Pyrrhotite Bridgmanite Serpentine Fougerite And Mackinawite Html
We're asked to build the orbital diagram for the ion most likely formed by phosphorus. This requires determining first the ground-state electronic configuration of phosphorus (P) by referring to the periodic table and locating the position of P. Ground state means that the element is in its lowest energy form (not in an excited state).
Electrons move around the atomic nucleus in specific regions, called orbitals. An orbital is a region of space that one or two electrons can occupy. Even though one or two electrons may occupy a given orbital, the most stable and balanced condition occurs when the orbital contains two electrons.
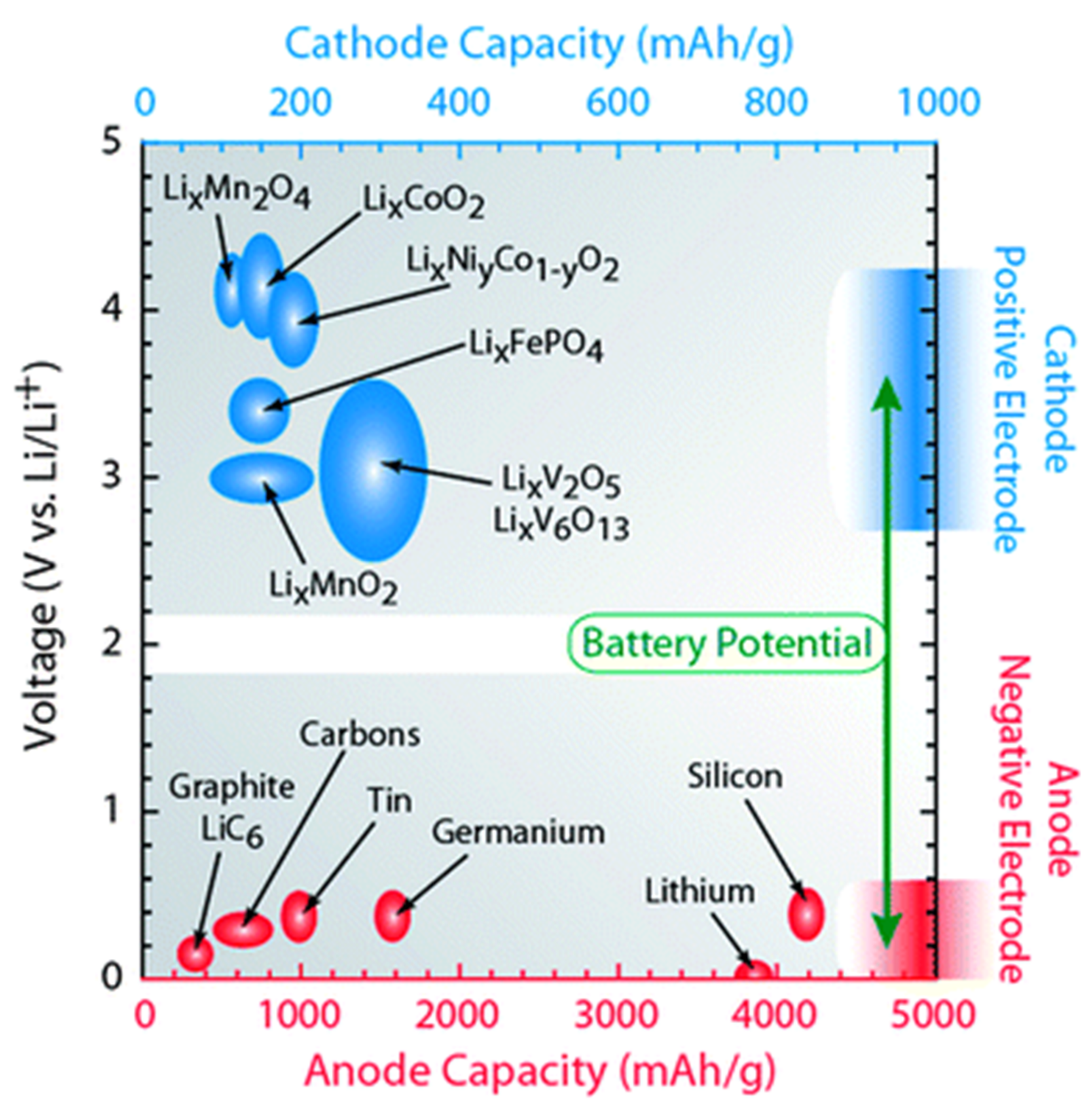
Nanomaterials Free Full Text Growth Mechanism Of Micro Nano Metal Dendrites And Cumulative Strategies For Countering Its Impacts In Metal Ion Batteries A Review Html
These 10 chemistry test questions deal with the concepts of electronic structure and configuration. draw the orbital notation C. the subshell of p orbitals _____ c. Consider the orbital diagram: 1. Build the orbital diagram for the ion most likely formed by phosphorus. What is the electron configuration of Iron? 66.
Part B:Build the orbital diagram for the ion most likely formed by phosphorus. Phosphorous forms an ion with a charge of -3, which means it has the same electron configuration as Argon. That electron configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6,

Pbs Qd Based Photodetectors Future Oriented Near Infrared Detection Technology Journal Of Materials Chemistry C Rsc Publishing

Recent Progress On 2d Magnets Fundamental Mechanism Structural Design And Modification Applied Physics Reviews Vol 8 No 3
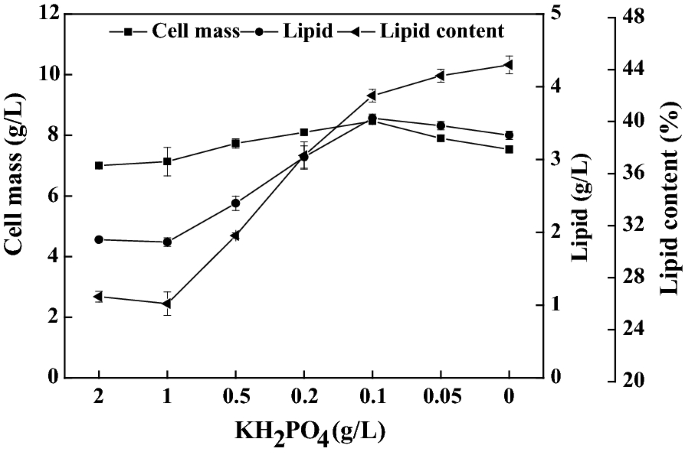
Phosphate Removal Combined With Acetate Supplementation Enhances Lipid Production From Water Hyacinth By Cutaneotrichosporon Oleaginosum Biotechnology For Biofuels Full Text
Solved Part 1 1 Point Write The Complete Ground State Conniguration For Phosphorus Part 2 1point What Are All Of The Possible Ions That Could Have The Following Electron Configuration 18282p 383p Choose
Full Configuration Interaction Simulations Of Exchange Coupled Donors In Silicon Using Multi Valley Effective Mass Theory Iopscience
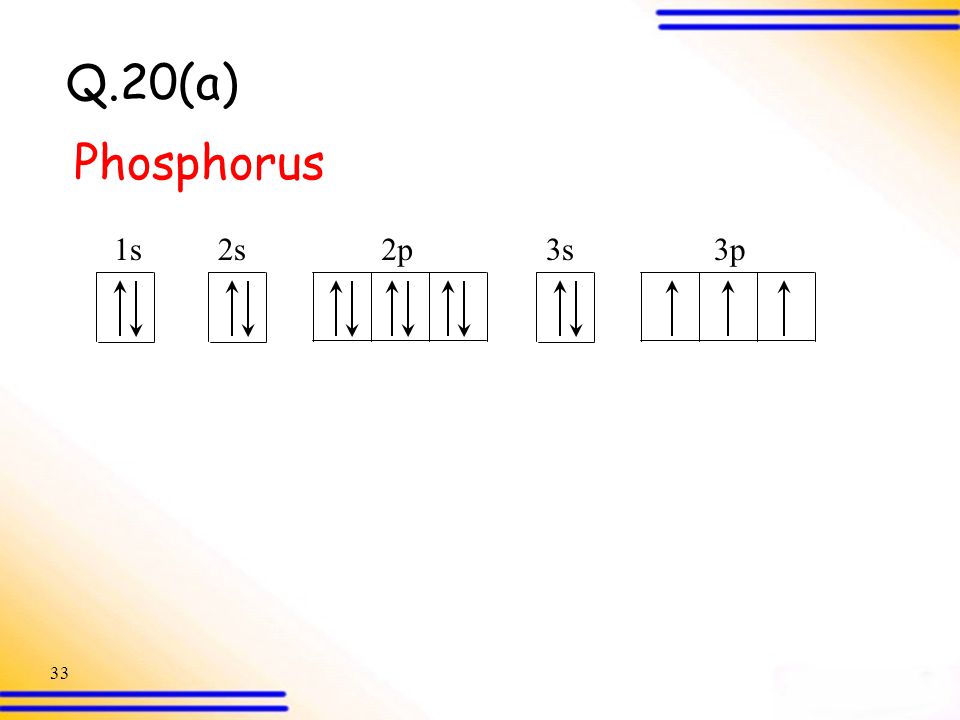
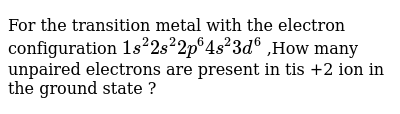











0 Response to "37 build the orbital diagram for the ion most likely formed by phosphorus."
Post a Comment