38 fluorine electron dot diagram
Lithium is the most stable element because it has to lose only one electron to achieve a stable configuration. Were going to do the lewis s... Examples: 1. Sodium (Na) and Chlorine (Cl). Electron Dot Diagram. Criss Cross Method. g. Fluorine _. d. Lead _. 4. Show the ionic bond that will occur using both methods: Magnesium and Phosphorus Electron Dot Diagram
Dot Diagram Fluorine N5 Electrical Schemes. Help With Lewis Diagrams High School Chemistry. Atoms Chemically Bond In An Attempt To Feel Stable Like. Lewis Structure For Of2 Oxygen Difluoride. What Is The Ionic Bonding Between Potassium And Fluorine. Dot Diagram Fluorine Wiring Diagram.
Fluorine electron dot diagram
Video: Fluorine Electron Configuration Notation. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. So that's fluorine, so we're going to start giving it, giving up the electrons of fluorine so we're going to say okay we have 20 of them 2, 4, 6, 8, 10, 12, 14, 16... When investigating any Electron Dot Diagram Of Fluorine wiring diagram, start by familiarizing by yourself Along with the symbols that are being used. The electrical symbols will likely not only display wherever anything is always to be put in, but what sort of unit is currently being installed.
Fluorine electron dot diagram. Fluorine electron configuration. ← Electronic configurations of elements. F (Fluorine) is an element with position number 9 in the periodic table. Located in the II period. Melting point: -219.6 ℃. Density: 0.00158 g/cm3. Electronic configuration of the Fluorine atom: 1s2 2s2 2p5. And thus the neutral atom has 7 valence electrons. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1... Fluorine Valence Electrons: Fluorine is a chemical element that has a symbol F. The atomic number of Fluorine is 9. As compared to the other electronegative element, it is totally reactive in nature. Here we have provided you the dot diagram of the fluorine valence electrons. Second we need to determine the center atom non hydrogen. Fluorine is in group 17 of the periodic table. Lithium Fluorine L...
Fluorine Electron Dot Diagram. Written By JupiterZ Tuesday, May 29, 2018 Add Comment Edit. Discussion 9. Dot Diagram Fluorine Wiring Diagram. See The Electron Configuration Of Atoms Of The Elements. Calcium Fluoride Caf2 Chemspider. Fluorine dot diagram Similiar electron dot structure for fluorine. See extra concepts about Fluorine dot … In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron. Lewis electron dot structures are the diagrammatic representation of covalently bonded atoms in a molecule. They also show lone pairs of electrons that may exist in the molecule. They are used in the case of covalently bonded molecules as well as coordination compounds. Study the electron dot diagrams for lithium, carbon, fluorine, and neon in Figure 6-1. Choose the statement that correctly identifies the most stable of the Typically atoms gain or lose electrons to gain. Stable electron configuration. Study the electron dot diagrams for lithium, carbon...
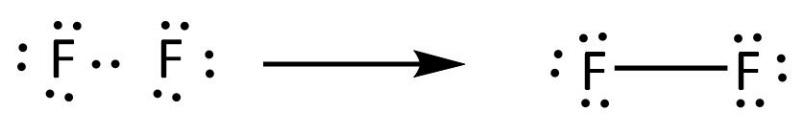
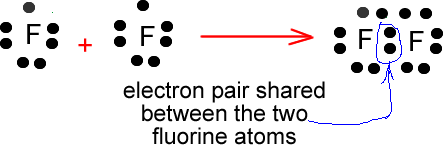
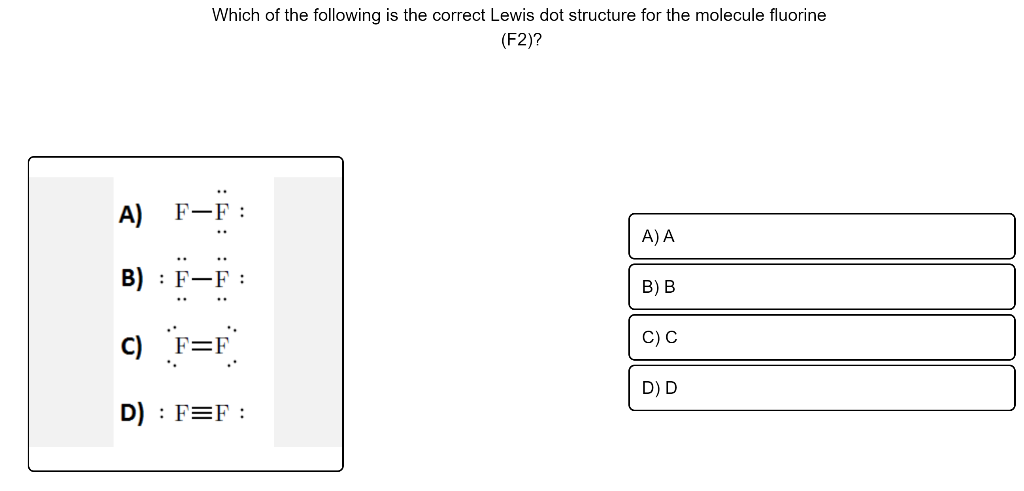
The electron dot diagram for helium, with two valence electrons, is as follows: By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. The diagrams provide predictions about the number of bonds. This is important, because it implies, among We can use the fluorine molecule as our first example. To diagram the F2 molecule, we start by drawing the Unlike a Lewis dot diagram, this figure does not portray electrons, but rather holes. Fluorine Orbital (Energy) Level Diagram. Valence electrons. Fluorine Atomic Structure (Bohr Model Diagram). What is it Commonly Used for. It is useful in the preparation of uranium hexafluoride to separate uranium isotopes in nuclear power plants. After that I draw the Lewis dot structure for Fluorine (F). Note: Fluorine is in Group 7 (sometimes called Group VII or Group 17). Since it is in Group 7 it will have 7 valence electrons.

Solved Chapter 6 Problem 13qp Solution Masteringchemistry Standalone Access Card For Basic Chemistry 3rd Edition Chegg Com
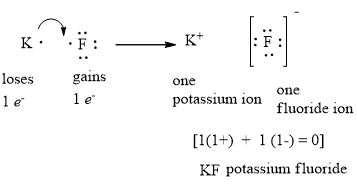
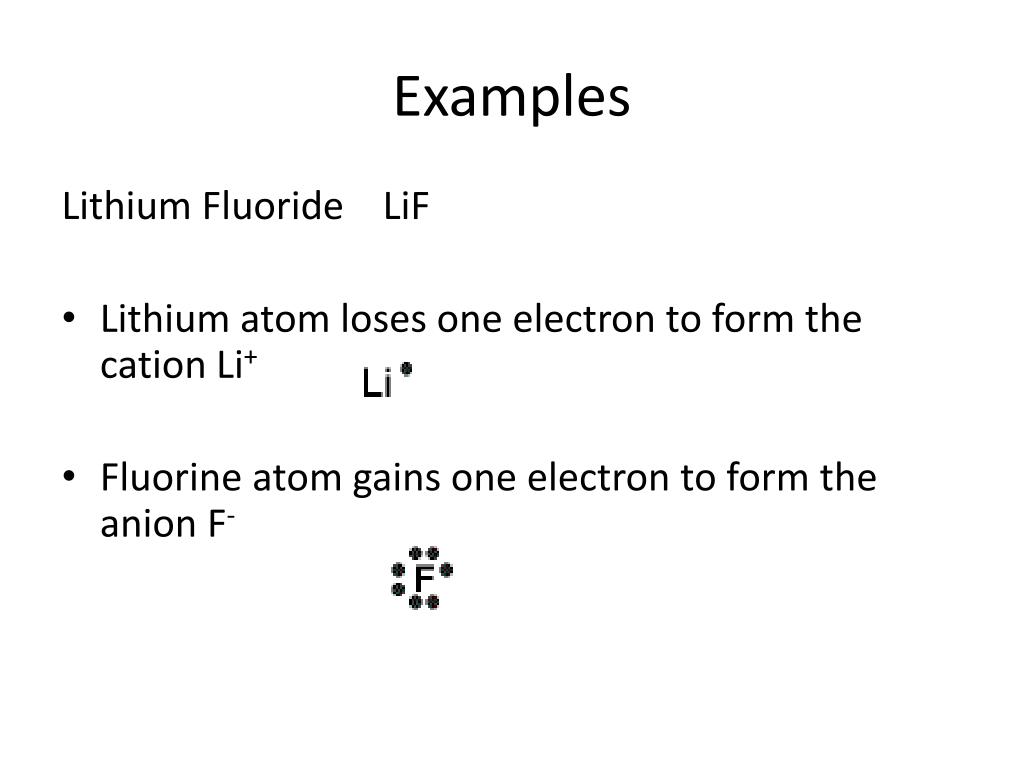
Electron Dot Diagrams. The electrons in an atom's outer energy level are the electrons that are important to consider in chemical bonds and chemical reactions. 1. Lithium atom loses one electron to form the cation Li+ 2. Fluorine atom gains one electron to form the anion F- 3. Lithium fluoride...
Lewis structure electron dot diagram for hydrogen fluoride or the 2 electrons making up the bonding pair of electrons between the hydrogen...
The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. This article gives an idea about the fluorine orbital diagram, period and groups, valency and valence electrons of fluorine, bond formation, compound formation, application of different principles.
And thus the neutral atom has 7 valence electrons. Socratic meta featured answers chemistry. The Octet Rule And Lewis Struc...
Electron dot diagrams sometimes called lewis dot diagrams were first used by gilbert n. So Boron can use those electrons. Once you have found the number of valance electrons place them around the elements symbol. Each Fluorine atom has 7 valence electrons.

Atomic Structure 1 Nucleus The Center Of The Atom And Is Made Up Off Protons And Neutrons 0 2 Electron Cloud Surrounds The Nucleus And Is Made Ppt Download
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that When doubling up electrons, make sure that a side has no more than two electrons. Fluorine and neon have seven and eight dots, respectively
Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second...
Fluorine's chemistry is dominated by its strong tendency to gain an electron. It is the most electronegative element and elemental fluorine is a strong Lewis dot diagram structures show three formal alternatives for describing bonding in boron monofluoride. Silicon tetrafluoride, similar to carbon...
2. Draw electron-configuration diagrams for the following atoms. (You will need to know each atom's electron. configuration; see Question 12 of the Shedding Light on Atoms Episode 6: Electron Shells worksheet.) Carbon, C. Nitrogen, N. Sulfur, S. Fluorine, F.
Electron Dot Diagram . Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surroundin...
Therefore the electron dot representation of fluorine atom is -F though it can also be represented by R, F or F ... [Pg.9]. Krypton is an inert gas element. Its closed-shell, stable octet electron configuration allows zero reactivity with practically any substance.
And well start looking on the periodic table. Study the electron dot diagrams for lithium carbon fluorine and neon in figure 6 1...
When investigating any Electron Dot Diagram Of Fluorine wiring diagram, start by familiarizing by yourself Along with the symbols that are being used. The electrical symbols will likely not only display wherever anything is always to be put in, but what sort of unit is currently being installed.
Lewis Electron Dot Diagrams are used to visually depict bonding by representing valence electrons as dots surrounding an elemental symbol. So that's fluorine, so we're going to start giving it, giving up the electrons of fluorine so we're going to say okay we have 20 of them 2, 4, 6, 8, 10, 12, 14, 16...
Video: Fluorine Electron Configuration Notation. The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.

Draw The Electron Dot Structures For A H 2 S B F 2 Sarthaks Econnect Largest Online Education Community




















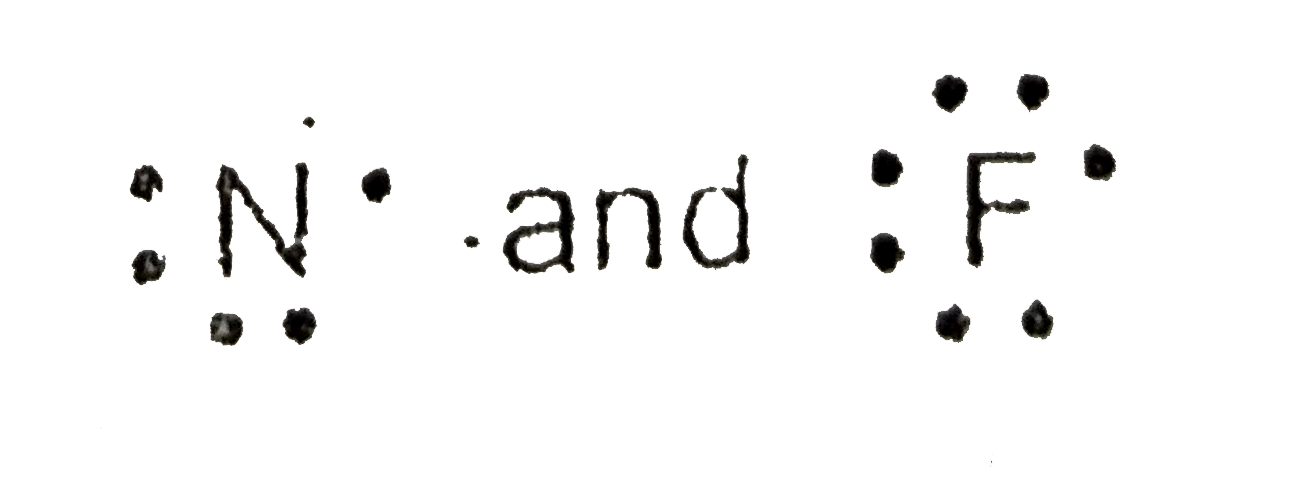

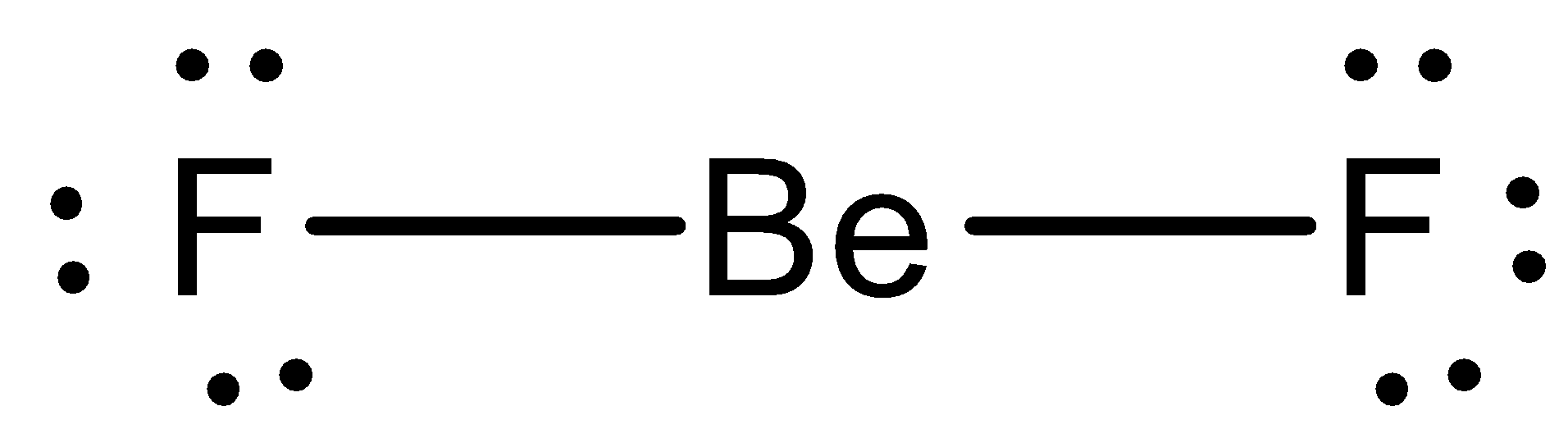






0 Response to "38 fluorine electron dot diagram"
Post a Comment