39 phase change diagram with equations
Teach Yourself Phase Diagrams A.6 HRS 03/11/2009 and Phase Transformations DEF.The equilibrium constitution is the state of lowest Gibbs free energy G, for a given composition, temperature and pressure. An alloy in this state shows no tendency to change – it is thermodynamically The phase line diagram has a close relative called a bifurcation dia-gram. The purpose of the diagram is to display qualitative information about equilibria, across all equations y′ = f(y), obtained by varying physical parameters appearing implicitly in f. In the simplest cases, each parameter change to f(y) produces one phase line diagram and
Thermodynamics of phase change 1 . T. HERMODYNAMICS OF PHASE CHANGE. ... Control Volume, was just a halt to change our view-point and see how the balance equations change when we let mass flows through the frontier of our system. Now we proceed with the thermodynamics of ... diagram is occasionally named Clapeyron diagram.
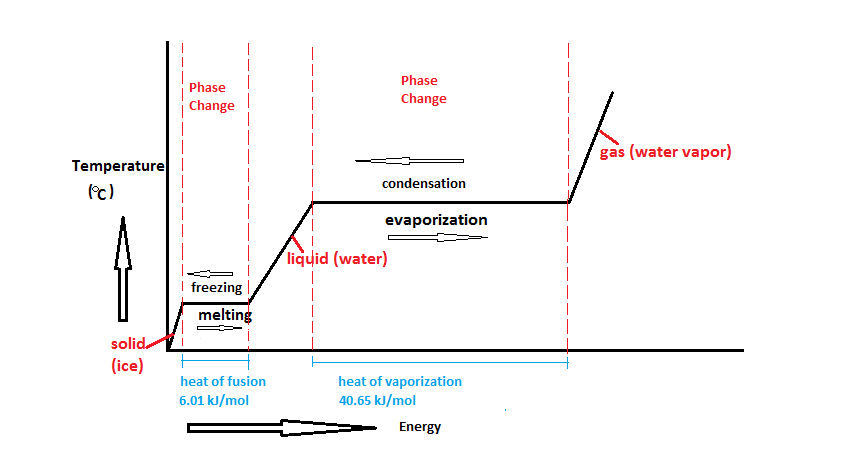
Phase change diagram with equations
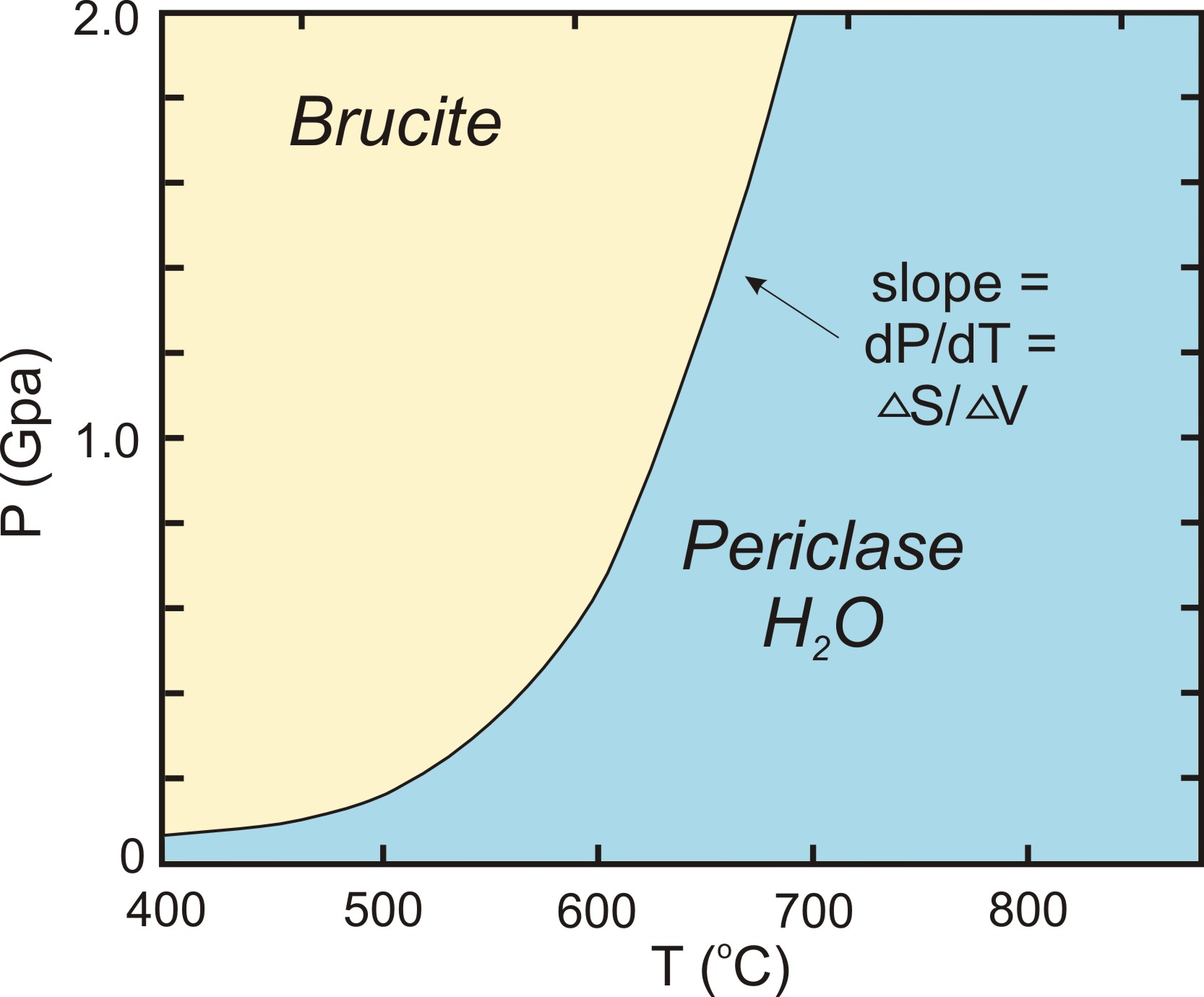
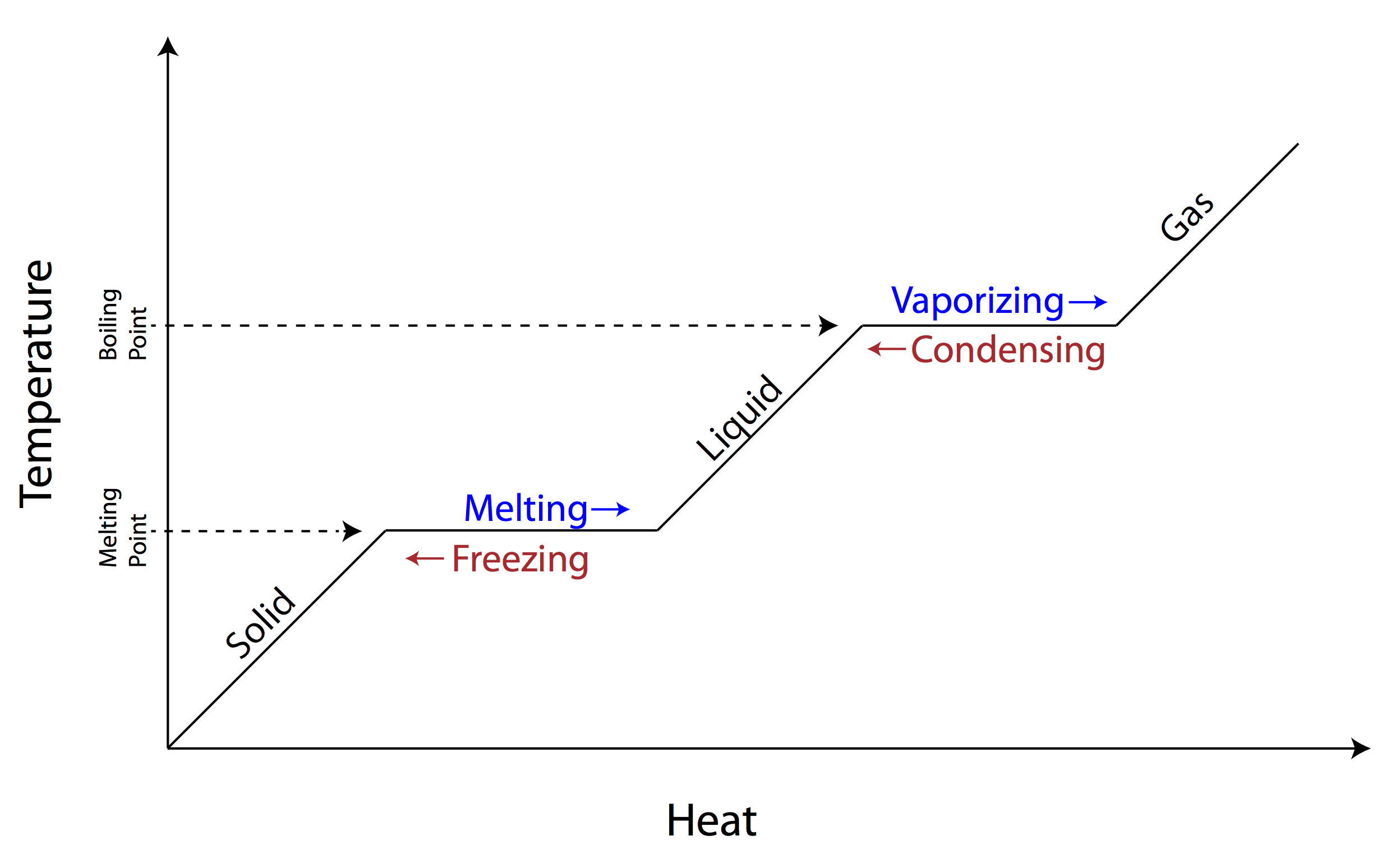
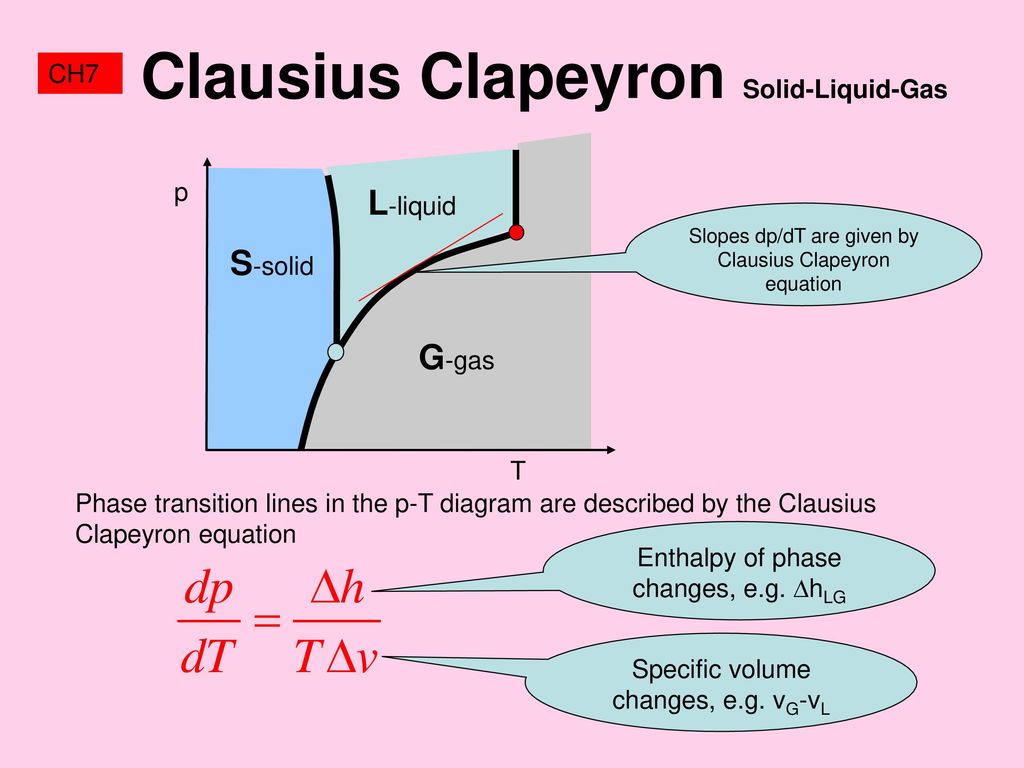
A phase diagram shows how temperature and pressure affect the phase of a substance ... Image showing the equations for each step of the phase change diagram. In this section we will give a brief introduction to the phase plane and phase portraits. We define the equilibrium solution/point for a homogeneous system of differential equations and how phase portraits can be used to determine the stability of the equilibrium solution. We also show the formal method of how phase portraits are constructed. The form of the Clapeyron equation most often used is dP/dT = Δ S/ Δ V This equations states that the slope (rise/run) of an univariant equilibrium plotted on a P-T diagram is equal to the entropy change (Δ S) of the reaction divided by the volume change (Δ V) of the reaction.Using the Clapeyron equation, only the slope of the equilibrium is determined, not the actual position of the ...
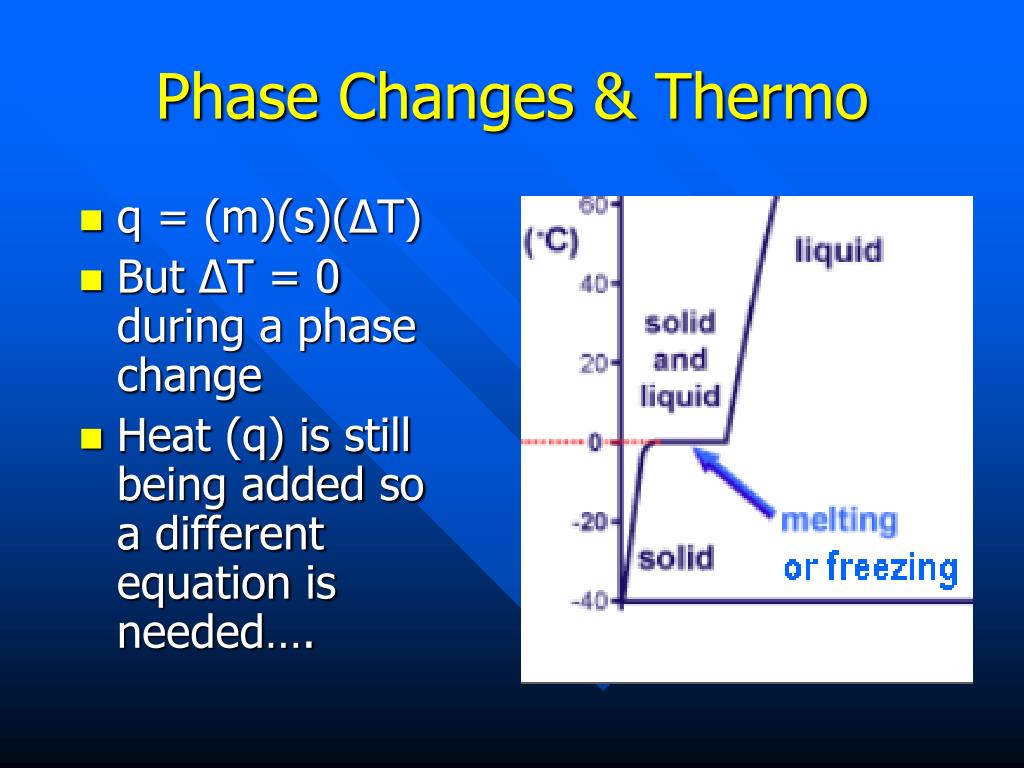
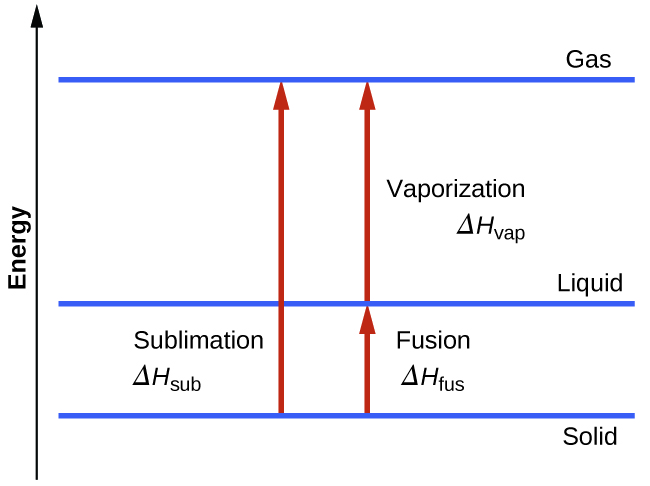
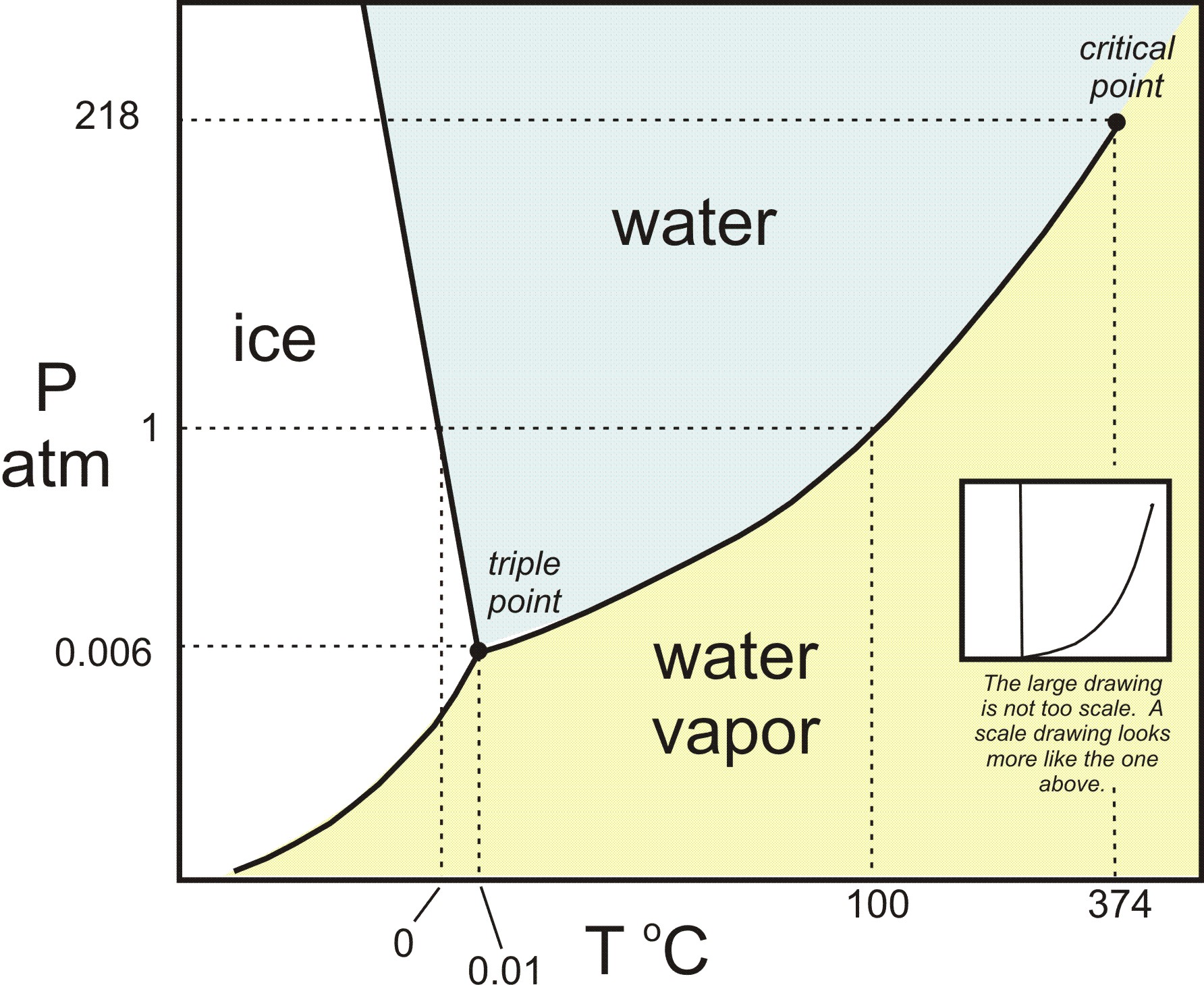
Phase change diagram with equations. MS15a, Gibbs Free Energy and Phase Diagrams 11/00 . The system can, in fact, lower its free energy even further by splitting up into a solid of composition X. S B. and a liquid of composition X. L B (shown on both diagrams). The gibbs free energy of the solid is given by point (4) on the g(X. B) diagram and that of the liquid by point (5) on ... Change of Phase Matters can be in four states like solid, liquid, gas and plasma. Distance between the molecules or atoms of the matter shows its state or phase . Temperature and pressure are the only factors that affect the phases of matter. Under constant pressure, when you heat matter, its speed of motion increases and as a result the distance between the atoms or molecules becomes larger. This is a summary of the equations to use to calculate phase transitions. The Clapeyron equation $\displaystyle p_2-p_1=\frac{\Delta H}{\Delta V}\ln\left( \frac{T_2}{T_1} \right)$ is used for a solid-liquid transition. The changes in enthalpy and volume relate therefore to changes occurring in fusion. Phase Diagrams. The phase of a given substance depends on the pressure and temperature. Thus, plots of pressure versus temperature showing the phase in each region provide considerable insight into thermal properties of substances. Such a pT graph is called a phase diagram. Figure 1.12 shows the phase diagram for water.
the diagram (phase fields) only one phase exists. Although a diagram of this kind delineates the boundaries of the phase fields, it does not indicate the quantity of any phase present. It is of interest to consider the slope of the liquid/solid phase line of the H. 2O phase diagram. 3.4 Solving Energy Problems Involving Phase Changes and Temperature Changes. When a cloud drop evaporates, the energy to evaporate it must come from somewhere because energy is conserved according to the 1 st Law of Thermodynamics. It can come from some external source, such as the sun, from chemical reactions, or from the air, which loses some energy and thus cools. Water Phase Diagram Sublimation, Saturation and Melting Lines Phase Diagram Data and Equations (Excel file) (68 KB. phase_diagram.xls) Phase Diagram Chart (PDF file) (8 KB. phase_diagram.pdf) Carbon Dioxide Phase Diagram Sublimation, Saturation and Melting Lines Phase Diagram Data and Equations (Excel file) (54 KB. co2_phase_diagram.xls) Phase Diagrams This common tangent construction can be extended to quite complicated situations, with several minima, which then give rise to quite complicated free energy curves and hence phase diagrams. For plotting a phase diagram we need to know how solubility limits (as determined by the common tangent construction) vary with temperature.
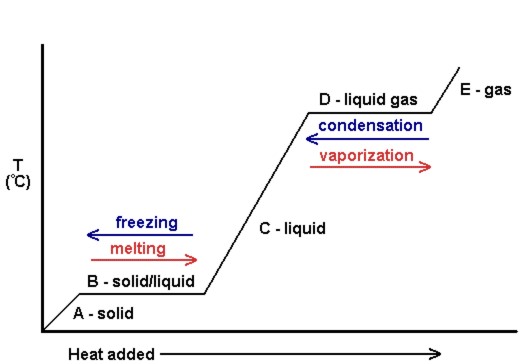
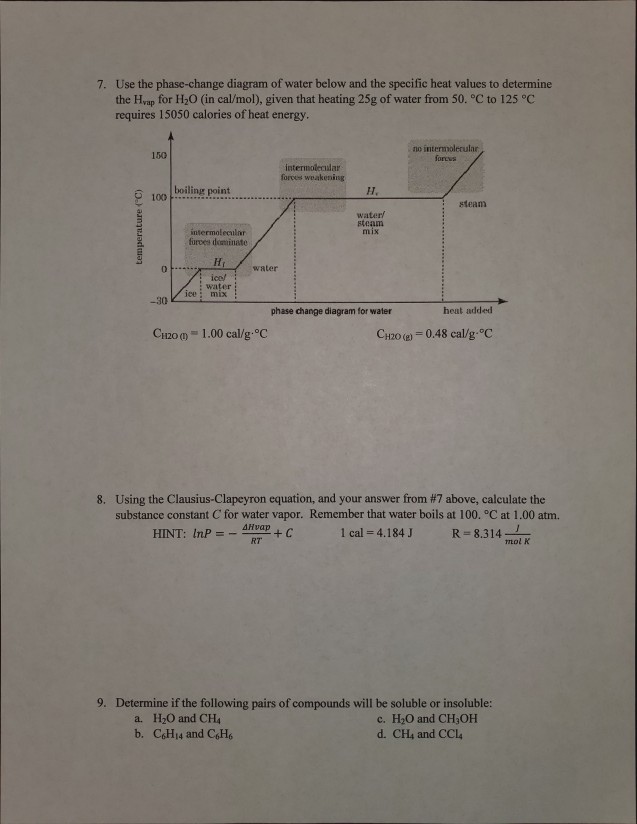
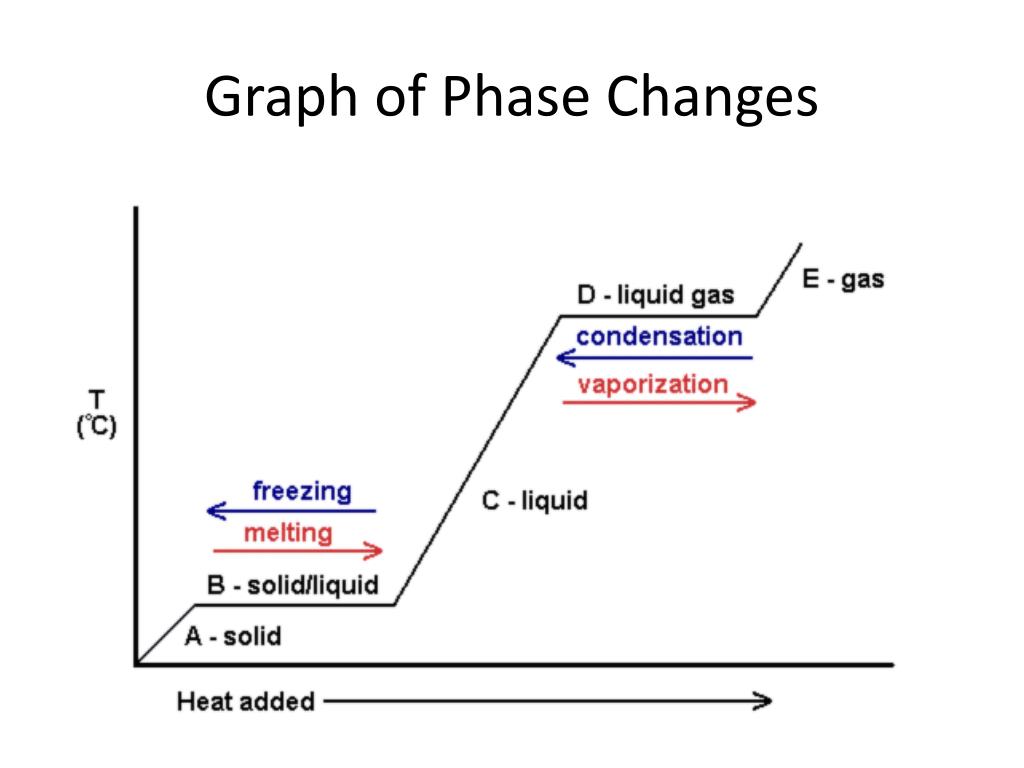
An equilibrium of such an equation is a value of x for which F(x) = 0 (because if F(x) = 0 then x'(t) = 0, so that the value of x does not change).. A phase diagram indicates the sign of x'(t) for a representative collection of values of x.To construct such a diagram, plot the function F, which gives the value of x'.For values of x at which the graph of F is above the x-axis we have x'(t) > 0 ... A phase line diagram for the autonomous equation y0= f(y) is a line segment with labels sink, source or node, one mark and label for each root yof f(y) = 0, i.e., each equilibrium; see Figure 15. A phase line diagram summarizes the contents of a direction eld and all equilibrium solutions. It is used to draw threaded curves across the graph window, Phase change is often shown in a diagram like the one below: Diagram of water phase changes When a substance is in a solid state, it can absorb a lot of energy in the form of heat until it hits ... PHASE PLANE DIAGRAMS OF DIFFERENCE EQUATIONS TANYA DEWLAND, JEROME WESTON, AND RACHEL WEYRENS Abstract. We will be determining qualitative features of a dis- crete dynamical system of homogeneous di erence equations with constant coe cients. By creating phase plane diagrams of our system we can visualize these features, such as convergence, equi-librium points, and stability. 1. Introduction ...
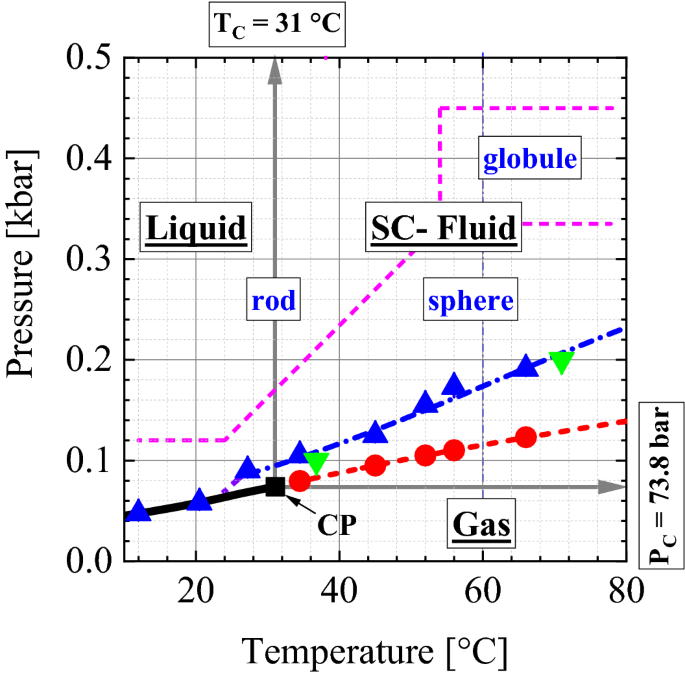
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
16 Feb 2021 — Phase transition is when a substance changes from a solid, liquid, or gas state to a ... Clausius-Clapeyron Equation · Phase Diagrams.Phase Changes · How Phase Transition works · Brief Explanation of a Phase...
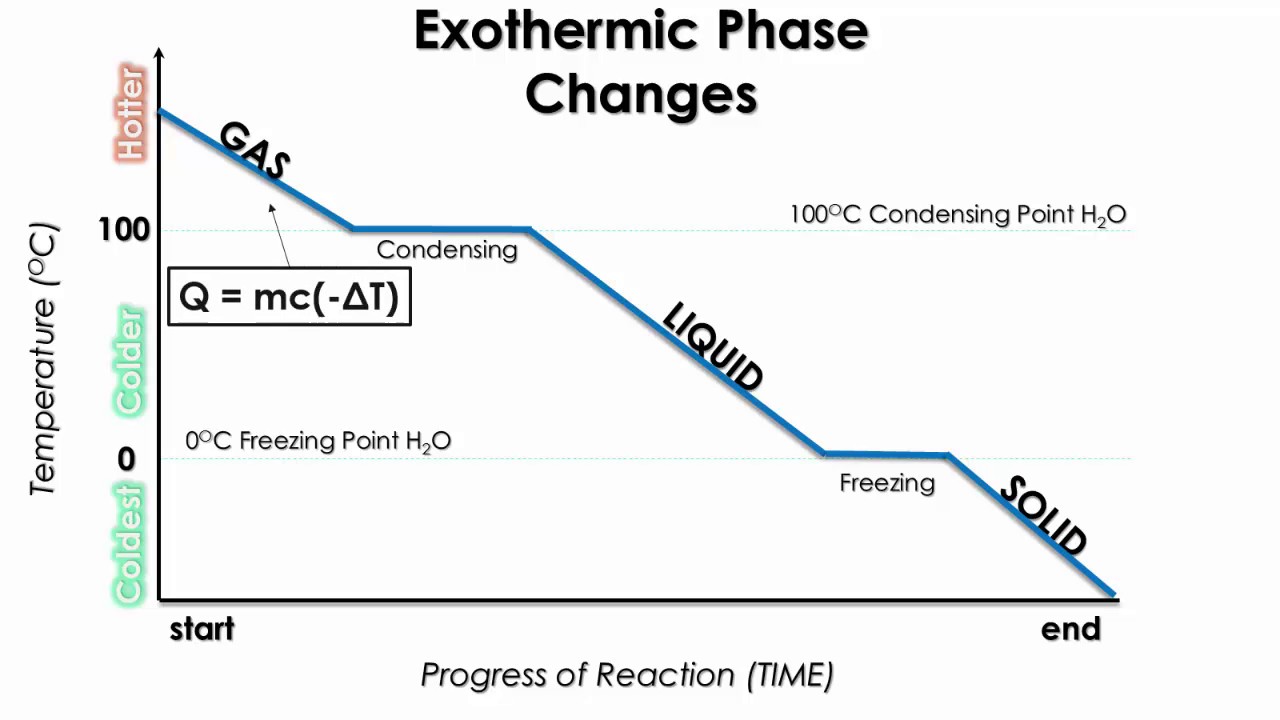
Using the equation for a change in temperature and the value for water (334 kJ/kg), we find that Q=mLf=(1.0kg)(334kJ/kg)=334kJ is the energy to melt a kilogram ...
A phase diagram is a graphical representation of the values of the thermodynamic variables when equilibrium is established among the phases of a system. Materials scientists are most familiar with phase diagrams which involve temperature, , and T composition as variables. Examples are T-composition phase diagrams for binary
3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation,
Clausius-Clapeyron equation. [tln29] Entropy of a supercooled liquid. [tex30] Coexistence line of continuous phase transition. [tex37] Latent Heat and response functions. [tex124] Heat capacity of vapor in equilibrium with liquid phase. [tex41] Discontinuous transition: change in internal energy. [tex123] Dry ice. [tex125] Abnormal phase ...
$\begingroup$ @HansLundmark, I discovered PPLANE yesterday. I checked the link you mentioned and also looked at the 3rd edition of the manual the authors mention on their site. But nothing appears there which shows a phase diagram like the one I want.
condensation, freezing, latent heat, sublimation. latent heat of fusion, latent heat of vaporization, melting, vaporization. phase change, phase diagram ...
variously called constitutional diagrams, equilib- rium diagrams, or phase diagrams. A single- component phase diagram can be simply a one- or two-dimensional plot showing the phase changes in the substance as temperature and/or pressure change. Most diagrams, however, are two- or three-dimensional plots describing the
The diagram you mostly find associated with different phases of a substance is the so-called "phase diagram". This diagram shows the regions of stability of different phases as a function of temperature and pressure. The interfaces between these regions will be the phase transition lines. The phase diagram for CO 2 is shown below.
Let me write that down. And there are many forms of phase diagrams. This is the most common form that you might see in your chemistry class or on some standardized test, but what it captures is the different states of matter and when they transition according to temperature and pressure. This is the phase diagram for water.
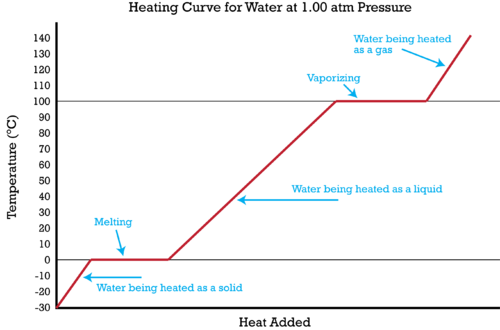
However, this equation cannot be used for phase changes, since temperature does not change during a phase change,as you can see in this diagram depicting water's phase changes: We know that the difference between any two phases is simply the energy difference, therefore all we need to know is that amount of energy to be able to calculate the heat involved. The equation used is q = m* DeltaH ...
Examples and explanations for a course in ordinary differential equations.ODE playlist: http://www.youtube.com/playlist?list=PLwIFHT1FWIUJYuP5y6YEM4WWrY4kEmI...
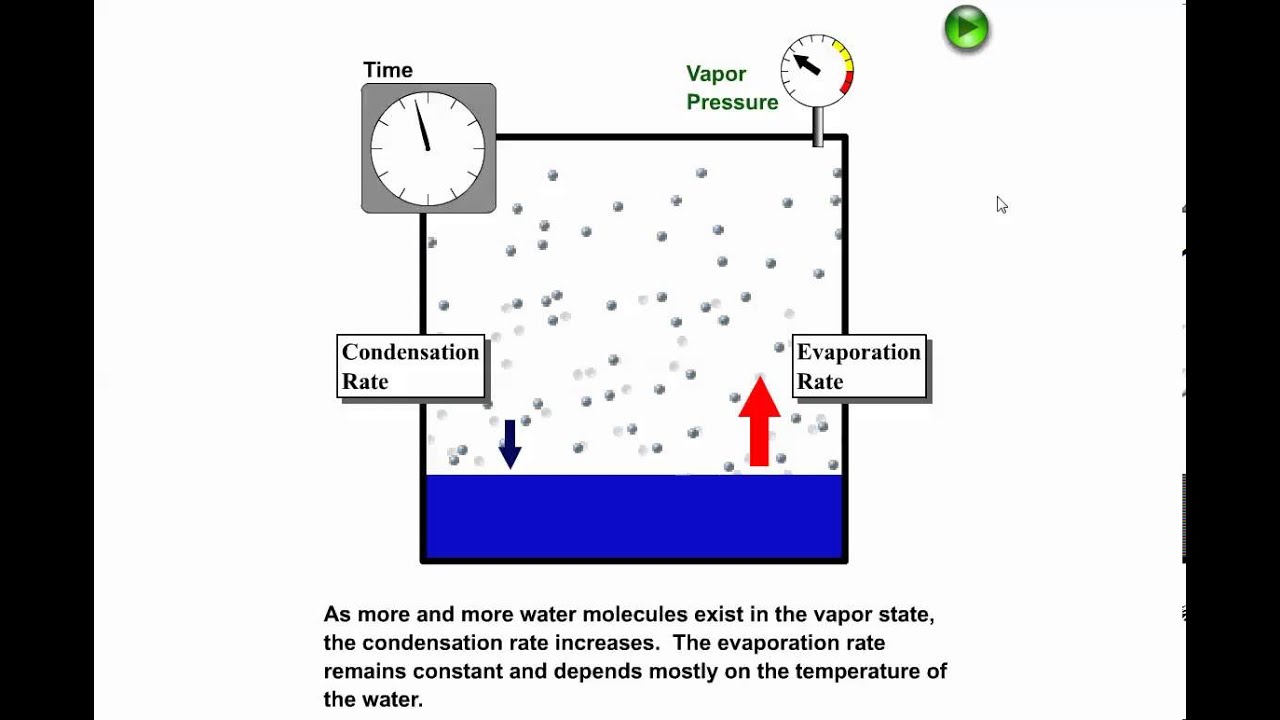
Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties. In the previous module, the variation of a liquid’s equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure ...
T-v diagram of constant-pressure phase-change processes of a pure substance at various pressures (numerical values are for water). 7 T-v diagram of a pure substance. 8 At supercritical pressures (P > P cr), there is ... substances in the gas phase is the ideal-gas equation of state.
The form of the Clapeyron equation most often used is dP/dT = Δ S/ Δ V This equations states that the slope (rise/run) of an univariant equilibrium plotted on a P-T diagram is equal to the entropy change (Δ S) of the reaction divided by the volume change (Δ V) of the reaction.Using the Clapeyron equation, only the slope of the equilibrium is determined, not the actual position of the ...
In this section we will give a brief introduction to the phase plane and phase portraits. We define the equilibrium solution/point for a homogeneous system of differential equations and how phase portraits can be used to determine the stability of the equilibrium solution. We also show the formal method of how phase portraits are constructed.
A phase diagram shows how temperature and pressure affect the phase of a substance ... Image showing the equations for each step of the phase change diagram.

Heat Phase Change Change Of Phase Change Of State The Term Change Of Phase Means The Same Thing As The Term Change Of State O These Changes Ppt Download

Quantification Heat And Temperature Can Be Measured And Quantified Through A Series Of Equations There Are 3 Thermometers 3 Temperature Scales We Can Ppt Download
















0 Response to "39 phase change diagram with equations"
Post a Comment