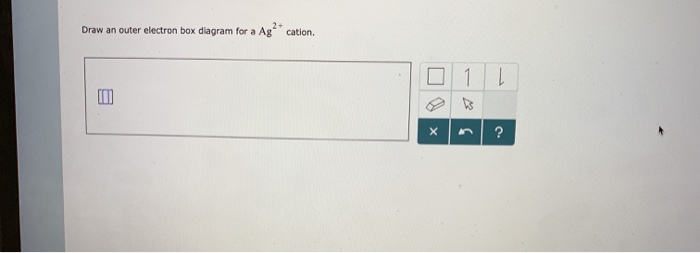
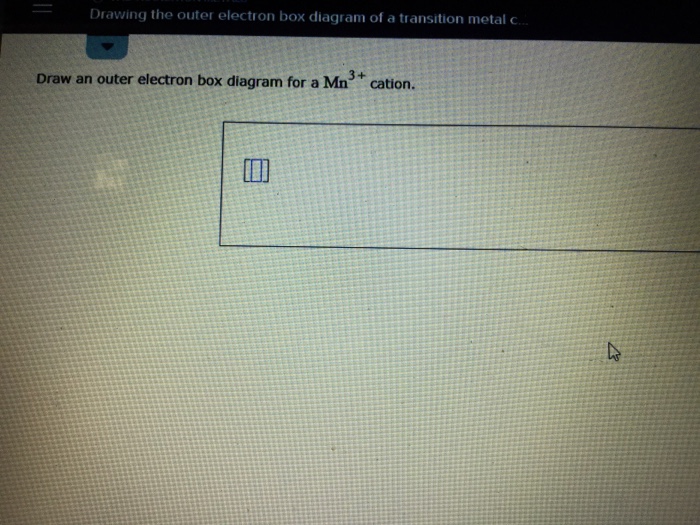
38 draw an outer electron box diagram for a cation.
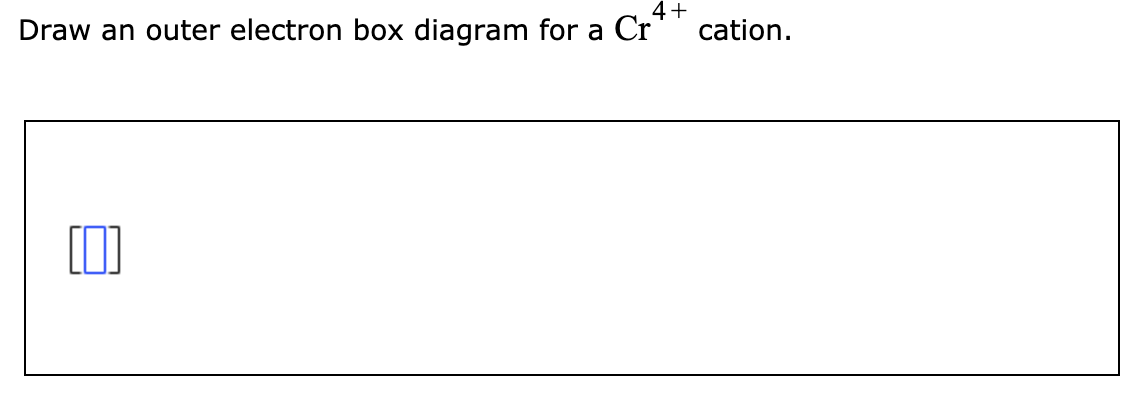
Science. Chemistry. Chemistry questions and answers. 4+ Draw an outer electron box diagram for a Cr* cation.
Lewis Dot Diagram Beryllium. This is the berylium chloride and boron chloride Lewis dot structure. Hydrogen, beryllium, and boron have too few electrons to form an octet. Beryllium Bohr Model The number of electrons in each of Beryllium's shells is [2, 2] and its electron configuration is [He] 2s2. The beryllium atom has a radius of.
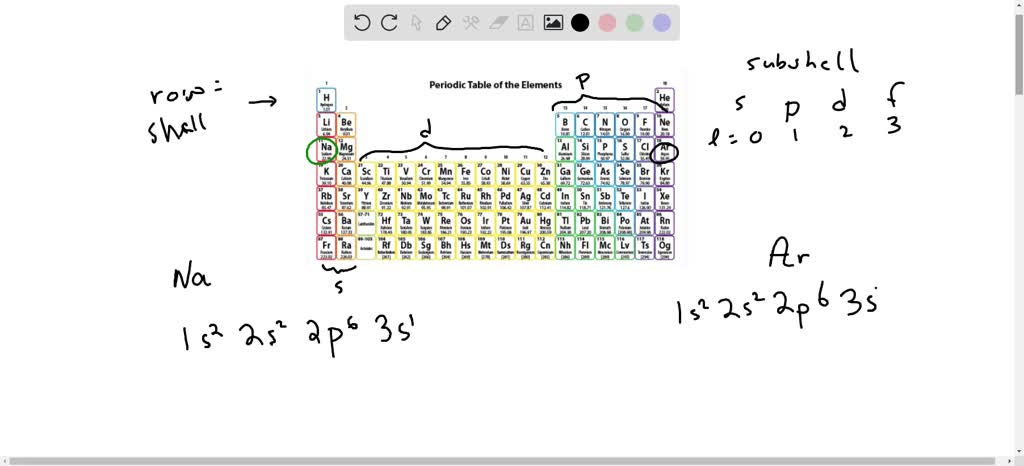
Therefore the Potassium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1. Video: Potassium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom.

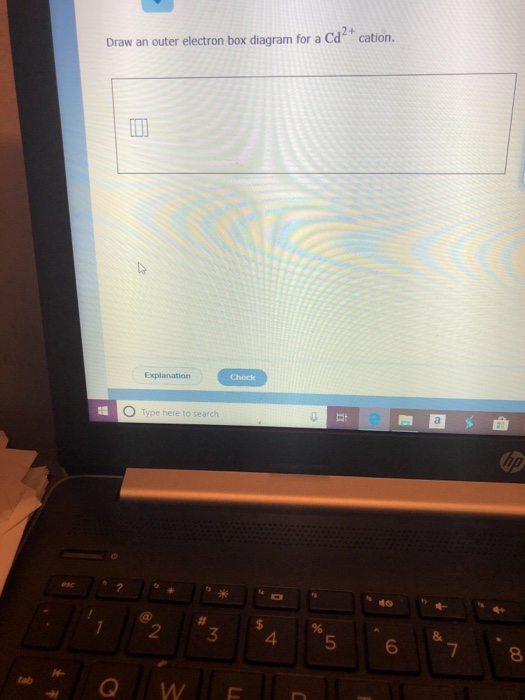
Draw an outer electron box diagram for a cation.
Orbital diagrams make use of a box, circle, or line for each ... same outer electron configuration. Elements in the same group of the periodic table exhibit ... gaseous atoms or ions. Atoms with a . low IE. tend to form . cations. Atoms with a . high IE. tend to form . anions (except the
A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus.
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Draw an outer electron box diagram for a cation..
cation: A positively charged ion, as opposed to an anion. valence shell: The outermost shell of electrons in an atom; these electrons take part in bonding with ...
(i) Construct two half-equations for this reaction to show electron loss and gain. [2] (ii) Draw a labelled enthalpy profile diagram for the overall reaction. On your diagram include † the enthalpy change of reaction, † the activation energy, † reactants, † products. [3] [Total: 10]
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ground state election configuration of Tc = [Kr] 4d5 5s2 S …. View the full answer. Transcribed image text: Draw an outer electron box diagram for a Tc cation. ク.
Predicting the ions formed by common main-group elements. Counting valence electrons in a ... Drawing a box diagram of the electron configuration of an atom.
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Rh2+ have 43 electrons. It's …. View the full answer. Transcribed image text: Draw an outer electron box diagram for a Rh cation.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next ...
Model Ill: Complex Lewis Dot Structures / Polyatomic Ions This! Use the atom cards and Cheerios to build the polyatomic ion before drawing it. Polyatomic ions are a group of atoms that are covalently bonded and act as a single unit with a charge. Building Lewis dot structures for polyatomic ions follows the same process as building a molecule.
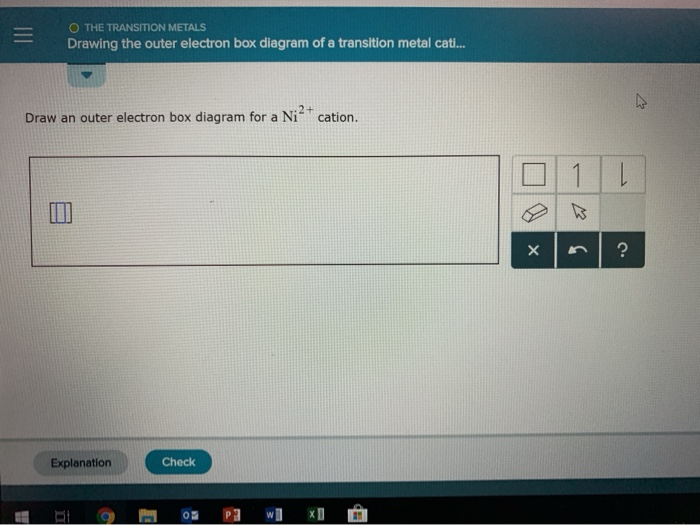
(5 points) (a) Write the short-hand electron configuration of Vanadium. (b) Draw the orbital diagram of the valence electrons in Vanadium. (c) Write the full ...1 answer · 0 votes: I Element 1 Atomic No. 1 22 Ni 28 # Cations are formed by loosing elections from ralence shells. Electronic configuration - 16² 25² 226 35² 3p% 3 d² ...
15 This question is about the properties of ions and ionic compounds. (a) Solid calcium carbonate, CaCO 3, has a giant ionic structure. (i) Draw a diagram (using dots or crosses) for a calcium ion. Show ALL the electrons and the charge on the ion. (2) (ii) Complete the electronic configuration for a calcium ion. (1) 1s2
And we have all of the P Electrons, all six of them filled for Argon. ... Drawing the outer electron box diagram of transition metal cation Draw an outer ...4 answers · Top answer: here. We're going to look at the ground state configurations for two elements sodium and our ...
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
Question: 4+ Draw an outer electron box diagram for a Nb" cation. Kr 4d 5s . This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text ... 4+ Draw an outer electron box diagram for a Nb" cation. Kr 4d 5s . Previous question Next question. COMPANY. About Chegg; Chegg For Good; College ...
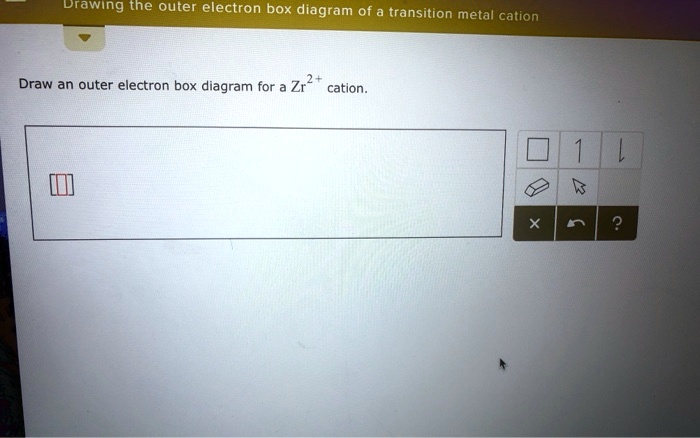
Solved Drawing the outer electron box diagram of a | Chegg.com. Science. Chemistry. Chemistry questions and answers. Drawing the outer electron box diagram of a transition metal cation Draw an outer electron box diagram for a Zrcation.
Model: Drawing Lewis Dot Structures for Atoms and Ions A. Lewis dot structure for an atom of chlorine is . The number of valence electrons for an atom is the number of electrons in the outer energy level (shell) of the atom. Chlorine's electron configuration is 2-8-7; therefore it has
An electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or 'shells', around the central nucleus.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Question: Draw a dot- and- cross diagram for magnesium fluoride, showing all electrons. Answer: Dot- and- cross diagram for ionic compound -- magnesium fluoride. Part 3: Dot- and- cross diagrams for simple molecules (covalent molecules). Recap - Covalent molecules are made up of two of more atoms of non- metals.
1.6.1c draw electron configuration diagrams of cations and anions using dots or crosses to represent electrons AND 1.6.2b draw electron configuration diagrams for simple covalently bonded molecules, including those with multiple bonds and dative covalent bonds, using dots or crosses to represent electrons
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
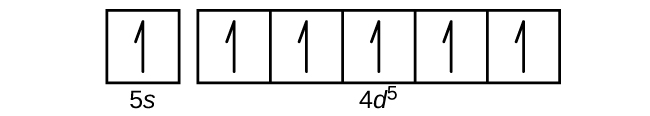
We're being asked to determine why is the Ru 3+ electron configuration [Kr] 4d 5 5s 0 instead of having a full s orbital.. Before we can do that, we have to first write the electron configuration of a neutral ground state Ruthenium (Ru).. You can determine the ground-state electron configuration of Ru by locating the position Ru in the periodic table.. Ground-state means that the element is ...
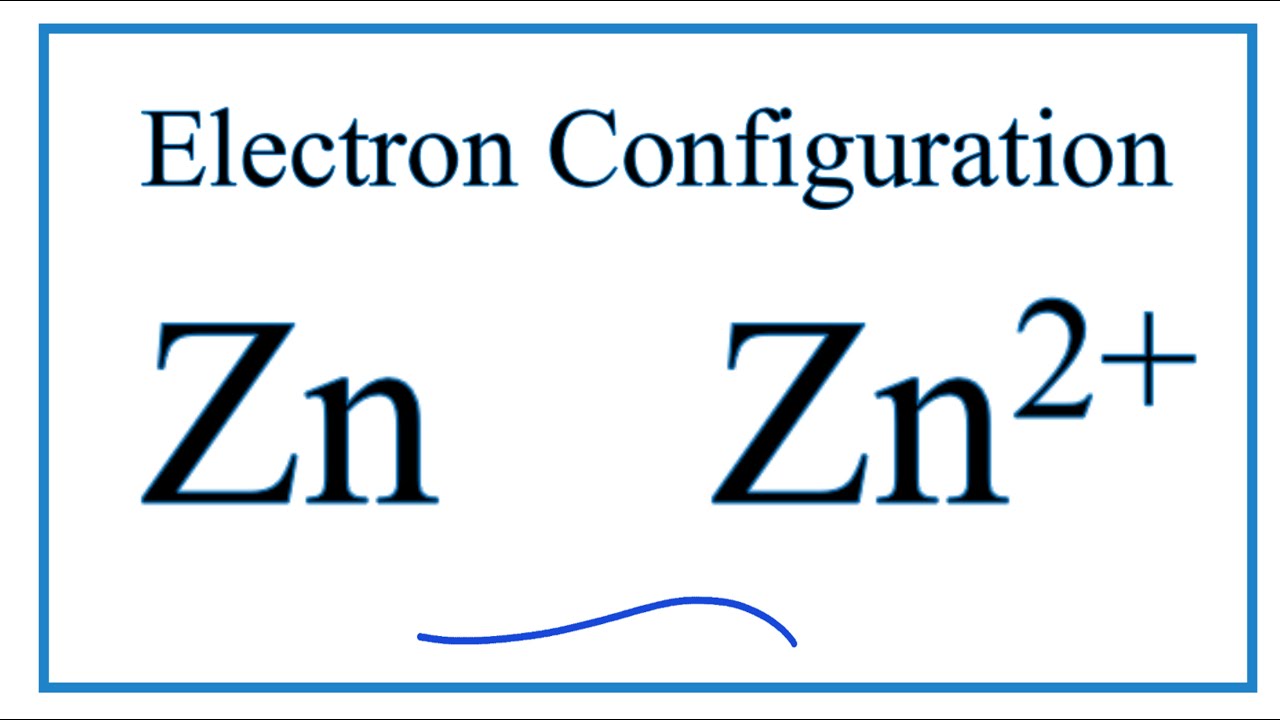
The electron configuration of "Zn"^(2+)" is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"3d"^10". Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates.
Using outer electron shells only, draw 'dot-and-cross' diagrams for molecules of BF3 and NH3. Use your diagrams to explain why a molecule of BF3 has bond angles of 120° and NH3 has bond angles of 107°.
Question: Draw an outer electron box diagram for a CO^2+ cation. This problem has been solved! See the answer ...
In order to write the Iron electron configuration we first need to know the number of electrons for the Fe atom (there are 26 electrons). Once we have the configuration for Fe, the ions are simple. When we write the configuration we'll put all 26 electrons in orbitals around the nucleus of the Iron atom. Video: Fe, Fe2+, and Fe3+ Electron ...
Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ... Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8.
Write the electron structure of the two cations. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse." Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Write the electron configurations for the following atoms ...
Answer to: Draw an outer electron box diagram for a [{MathJax fullWidth='false' Mo^{2+}}] cation. By signing up, you'll get thousands of...1 answer · Top answer: The electronic configuration of Mo is: [Kr]4d55s1[Kr]4d55s1 Electronic configuration of {eq}Mo^{2+}: \left [ Kr ight...
Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram.
Drawing the outer electron box diagram of transition metal cation draw an outer electron box diagram for zr2 cation 72237









![Why is Ru3+ electron configuration [Kr] 4d5 5s0 instead of having a full s orbital?](https://cdn.clutchprep.com/video_thumbnails/38938.jpg)








0 Response to "38 draw an outer electron box diagram for a cation."
Post a Comment