40 orbital diagram of as
Jan 01, 2022 · Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na) An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals.
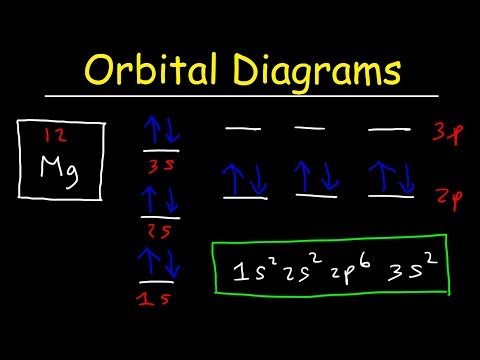
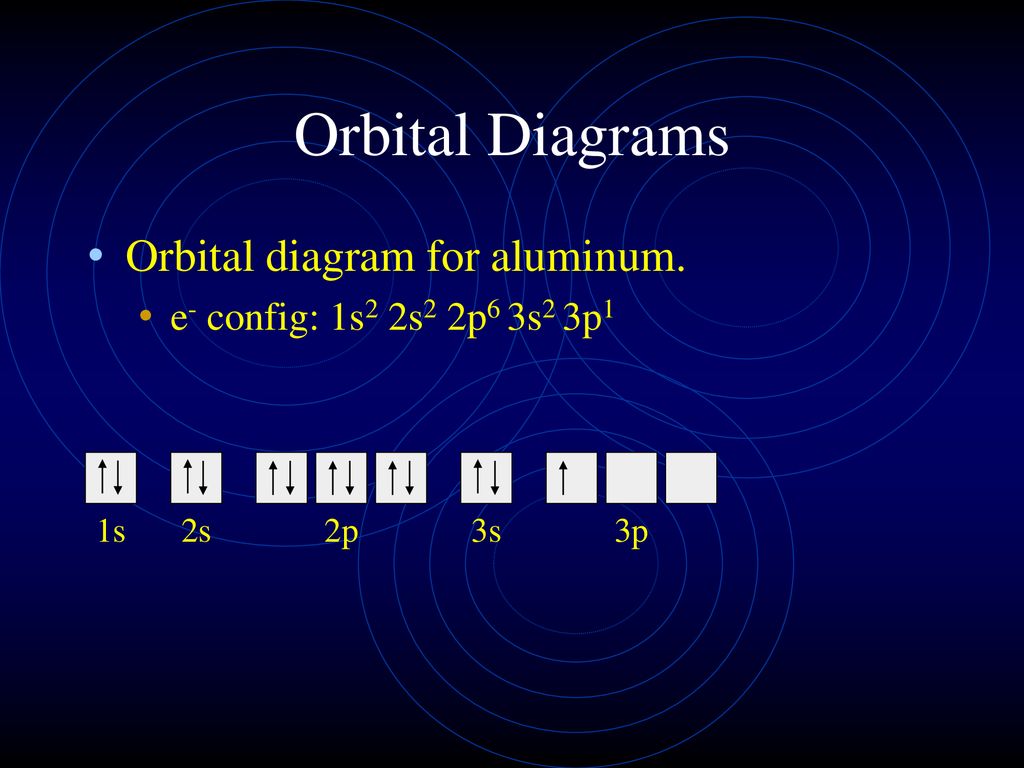
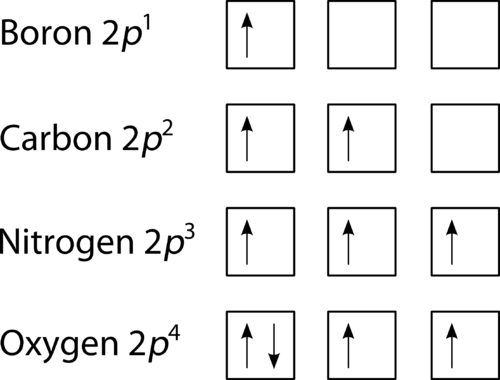
Its electron configuration is 1s22s22p2. The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with opposite spins (Pauli's exclusion principle).
Orbital diagram of as
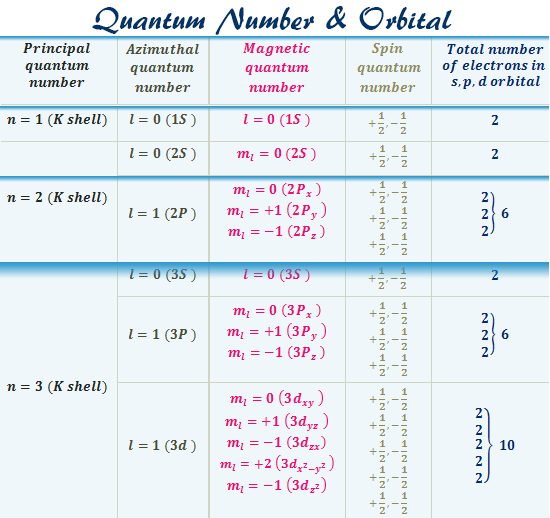
An s-orbital is spherical with the nucleus at its centre, a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. The orbitals in an atom are organized into different layers or electron shells. Oxygen (O) electron configuration with full orbital diagram. Oxygen (O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is 'O'. The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen ... A qualitative molecular orbital diagram for ferrocene (D 5d) FeII Fe SALC's p p a 2u * e 1u * x e 1u z y a e 2g * e 2g, u a 2u, e 1u a 1g * u a 1g e 1g * LUMO a 1g, e 1g, e 2g e 2g a 1g HOMO dyz dxz e 1g e 1g e 1u e 1g, e 1u a 1g a 2u a 1g, a 2u
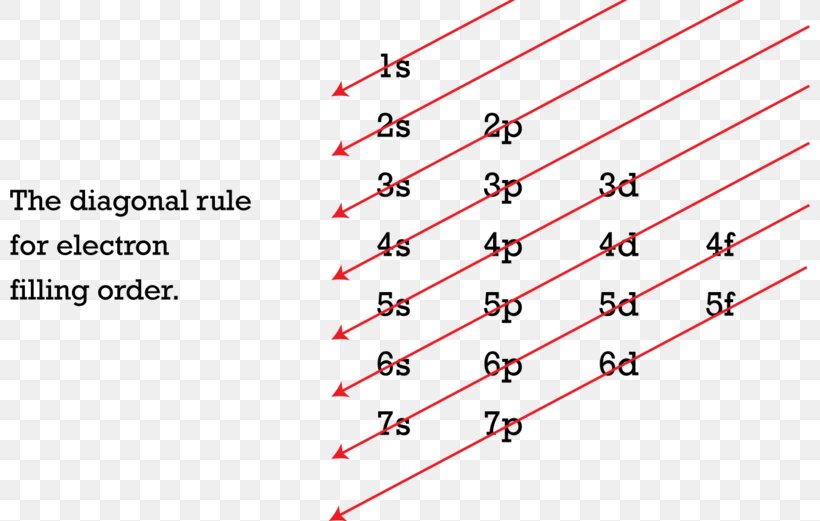
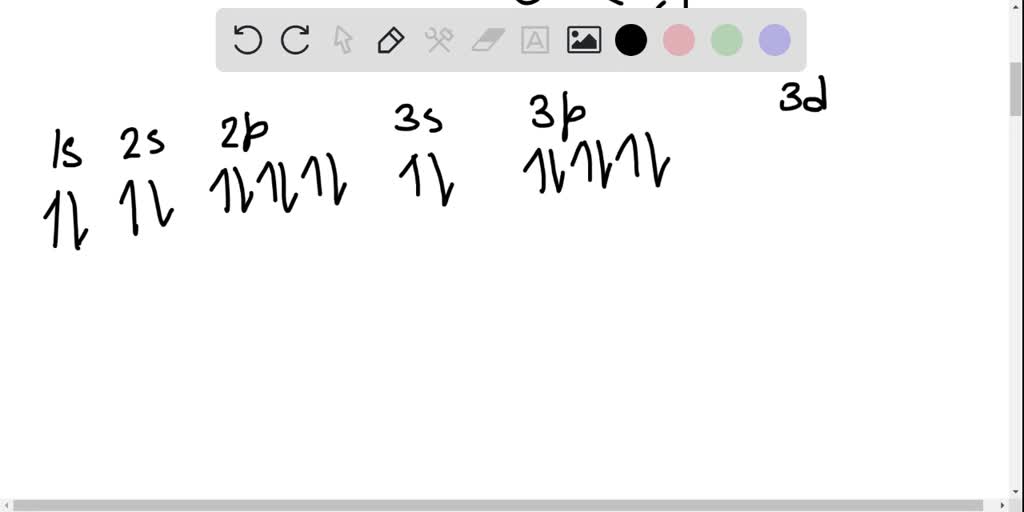
Orbital diagram of as. 28 Jan 2021 — Arsenic Electron Configuration (As) with Orbital Diagram ... Arsenic Electron Configuration: Chemical element, Arsenic has atomic number 33 and ... The MO diagram is the diagram as a whole. A molecular orbital diagram, Beryllium has an electron configuration 1s 2 2s 2, so there are again two electrons in the valence level. However, the 2s can mix with the 2p orbitals in diberyllium, whereas there are no p orbitals in the valence level of hydrogen or helium. The orbital filling diagram of ... An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s → 2s → 2p x 2p y 2p z → 3s → 3p x 3p y 3p z → Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
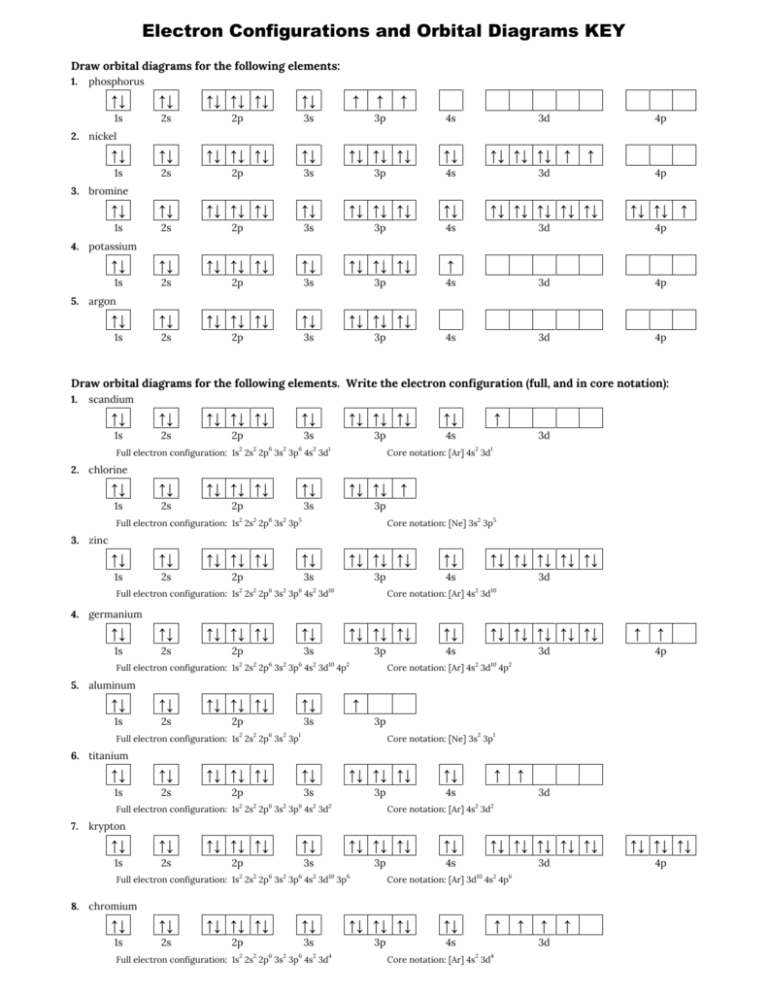
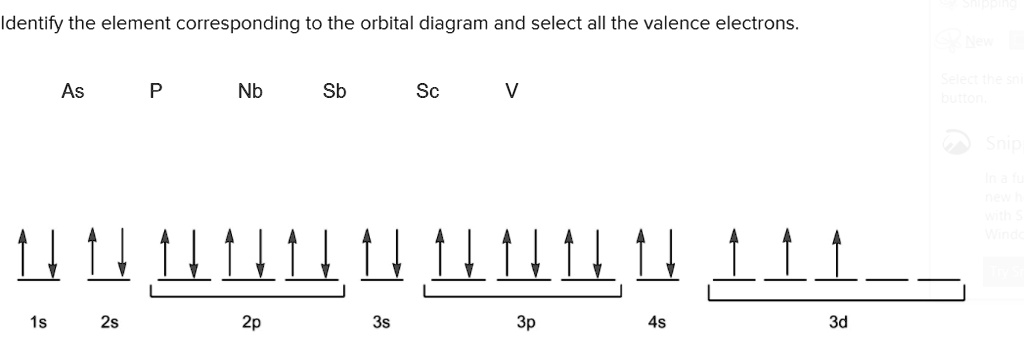
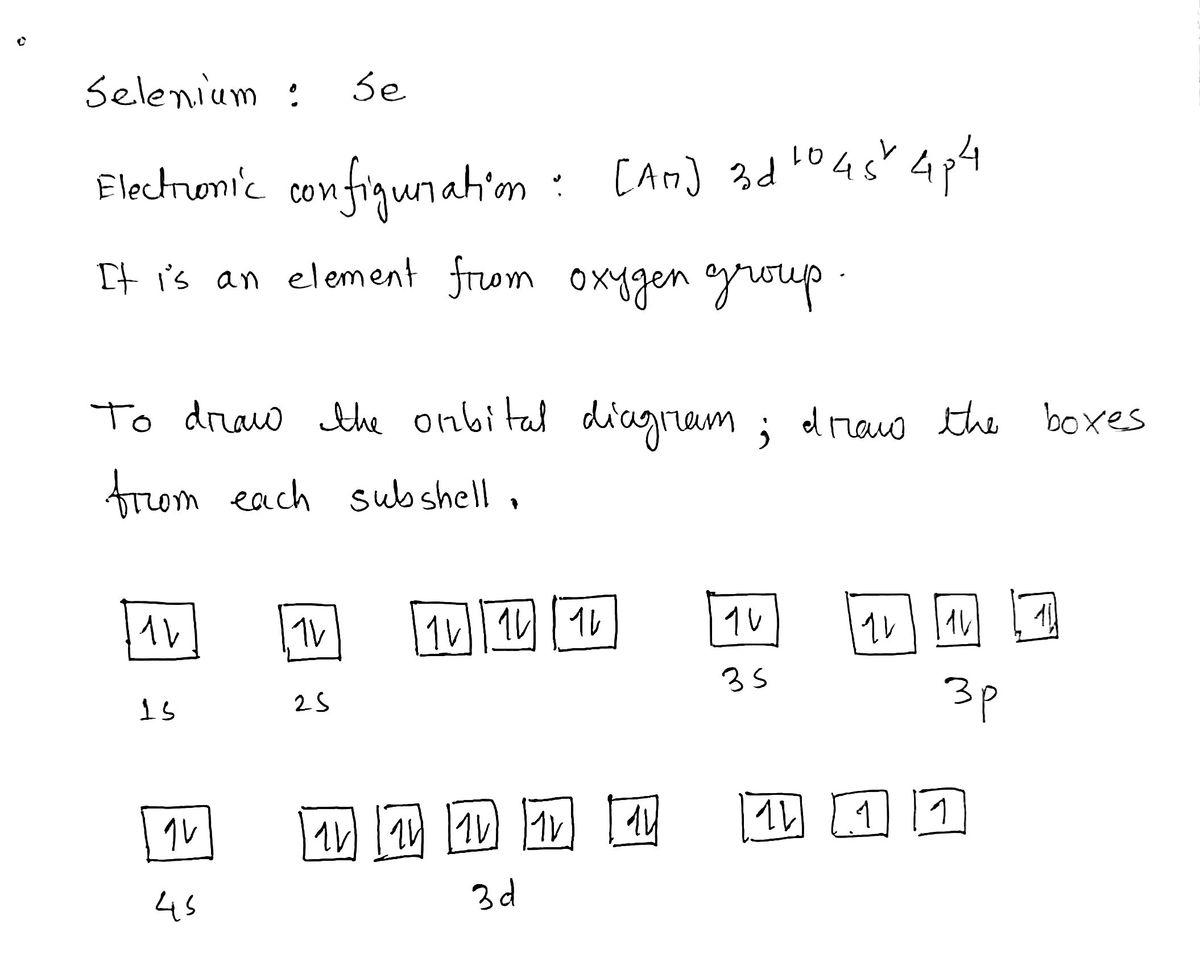
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s23p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in itsoutermost shell. schematron.org! Arsenic atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Write out configurations and draw orbital diagrams for the following. The remaining four electrons will go in the 2p orbital. We draw a molecular orbital energy diagram similar to that shown in figure 11. Molecular orbital diagrams bond order and number of unpaired electrons draw the molecular orbital diagram for the oxygen molecule o 2. 8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ...
4 days ago — Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. The wave functions, level energies and Mülliken population analysis of localized molecular orbitals (LMO's) for B4Cl4, 1,5-C2B3H5 and the closo-BnH2-n (n = 6-10, 12) are calculated by using the ... Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . What is an orbital energy diagram? Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Molecular Orbital Diagrams of more complicated molecules XH 2 (D ∞ h) H--X--H molecular orbitals general MO diagram layout linear central atom's (X 's) atomic orbitals atomic orbitals of terminal atoms H 1 + H 2 a linear combination of symmetry adapted atomic orbitals (LGO's)
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital.
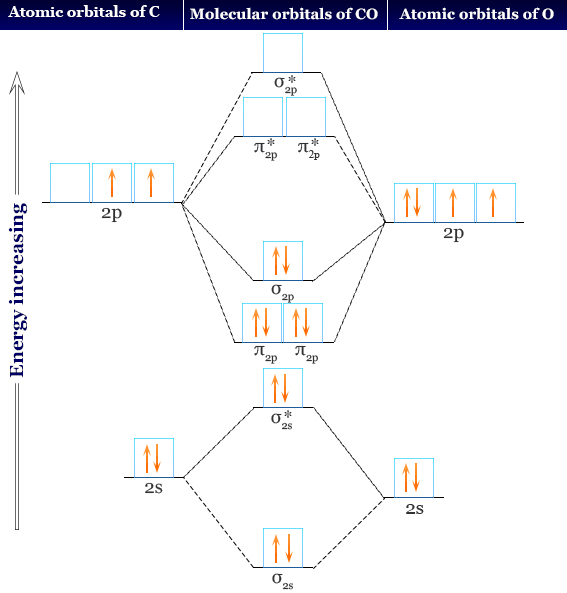
Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Mar 28, 2018 · Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
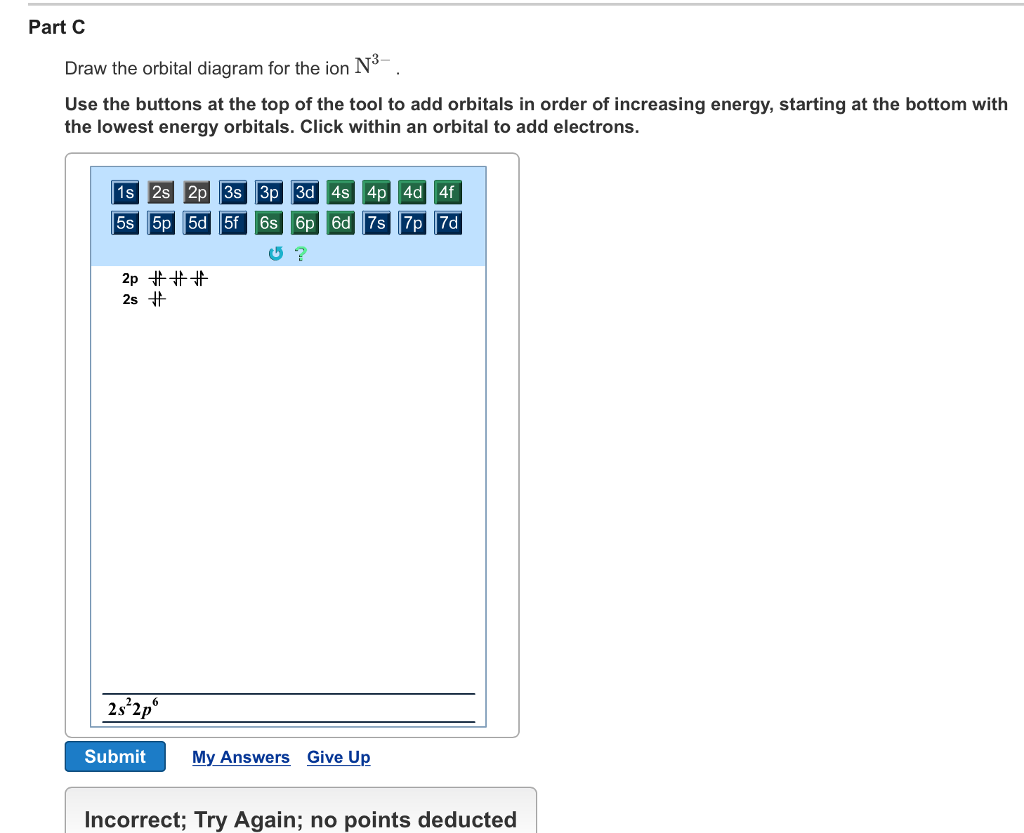
30 May 2020 — 1.4: Electron Configurations & Electronic Orbital Diagrams (Review) ... Before assigning the electrons of an atom into orbitals, ...
The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps!
Sodium(Na) is the 11th element in the periodic table and its symbol is 'Na'. This article gives an idea about the electron configuration of sodium and orbital diagram, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this.
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. The Pauli Exclusion Principle says that only two electrons can fit into an single orbital. I am fairly sure the first diagram I drew for carbon dioxide is wrong in terms of showing π bonding.
Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ...
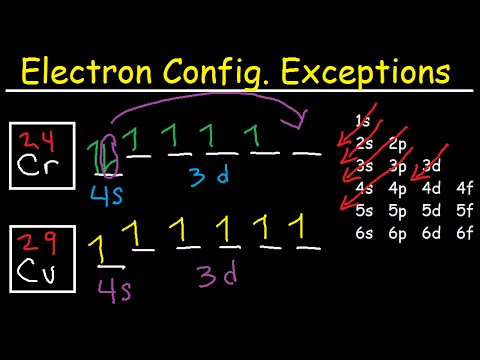
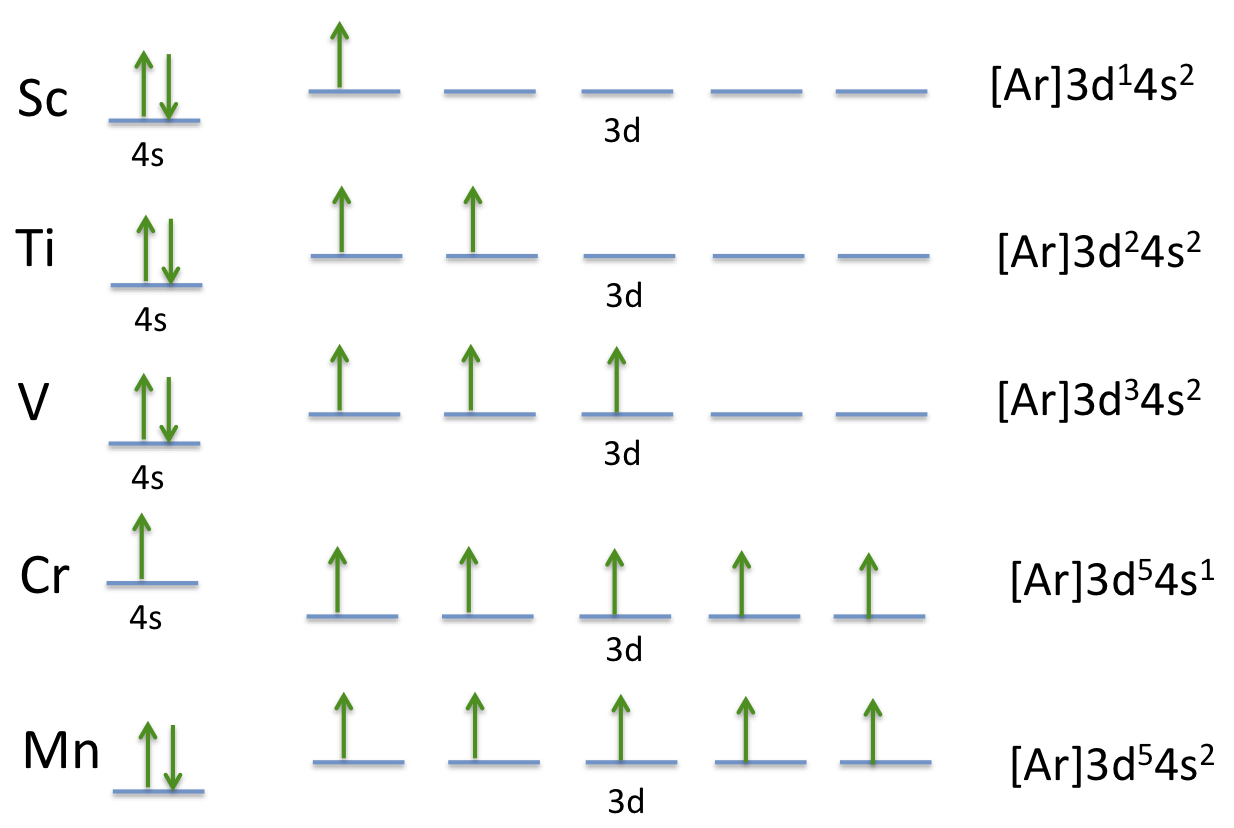
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means.
It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ...
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
A qualitative molecular orbital diagram for ferrocene (D 5d) FeII Fe SALC's p p a 2u * e 1u * x e 1u z y a e 2g * e 2g, u a 2u, e 1u a 1g * u a 1g e 1g * LUMO a 1g, e 1g, e 2g e 2g a 1g HOMO dyz dxz e 1g e 1g e 1u e 1g, e 1u a 1g a 2u a 1g, a 2u
Oxygen (O) electron configuration with full orbital diagram. Oxygen (O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is 'O'. The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen ...
An s-orbital is spherical with the nucleus at its centre, a p-orbitals is dumbbell-shaped and four of the five d orbitals are cloverleaf shaped. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. The orbitals in an atom are organized into different layers or electron shells.



















0 Response to "40 orbital diagram of as"
Post a Comment