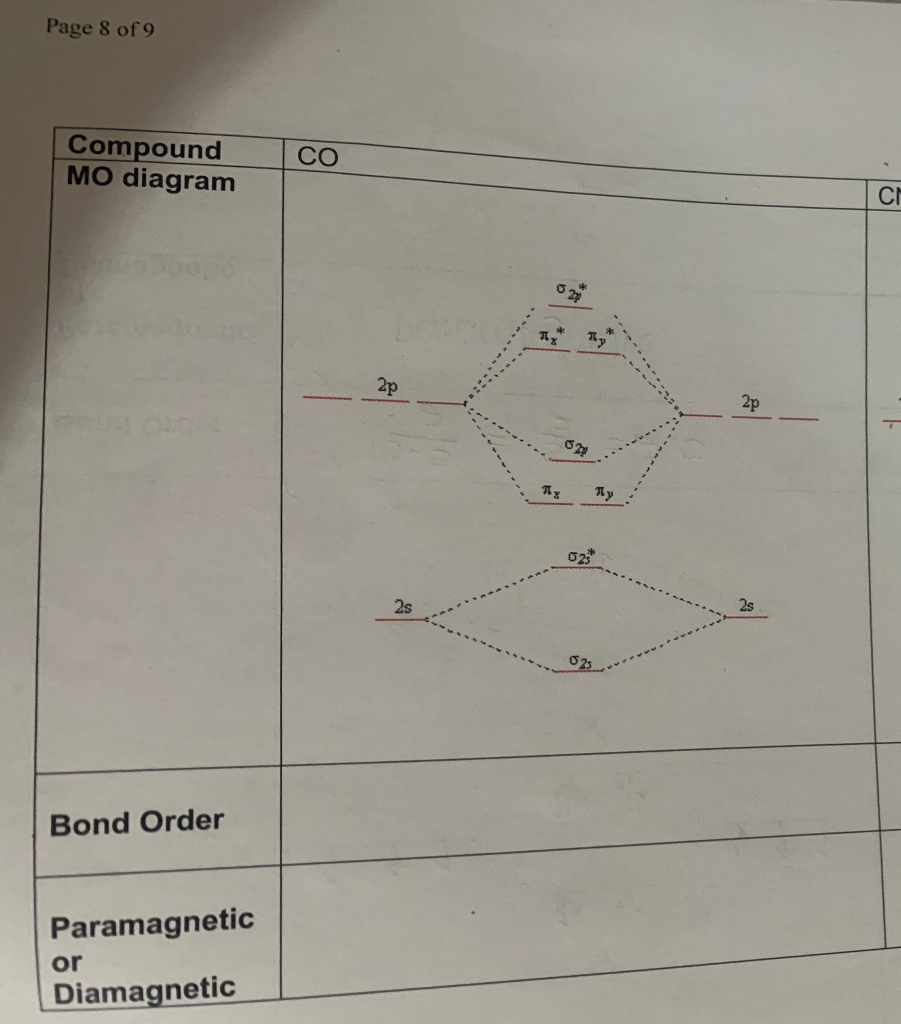
37 mo diagram for co
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row I know holy shit we are all excited asf. My tits ache too. But we are only close to the end when we are seeing mad price movents, trading haults and then i believe msm will change there tune completely when its over so they dont come across like the idiots they are, plus we haven't even started to see the margin calls fail. All these "this is it" "last chance to buy" is only going to discourse newer apes when nothing happens or we get a rug pulled again. Cuz its dam possible. Its happened mult...
The MO diagram for #"CO"# is The last electron is in fact in the #2b_1# antibonding MO, so the bond order of #"NO"# has decreased by #1/2# relative to #"NO"^(+)# or #"CO"#.

Mo diagram for co
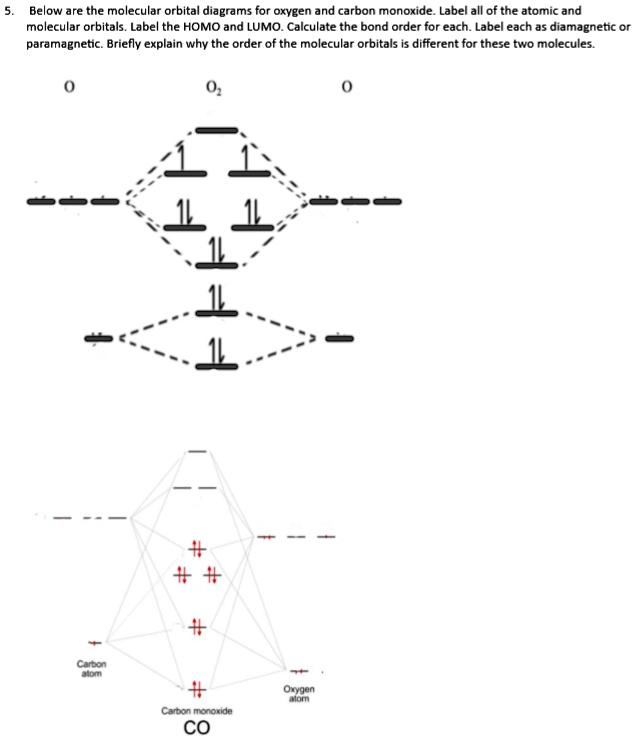
In the MO diagram for CO 2 in problem #36 , the stretched O 2 molecular orbitals have very little energy difference ( E). Why is the energy difference between the MOs in stretched O much smaller than the energy difference between the same MOs in non-stretched O 2 ? 40. For each species below... 2. Electronic configuration of CO molecule is Draw the MO diagram for acetylide ion C2^2- and calculate its bond order. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Mo diagram for co. Bonding in some heteronuclear di - atomic molecules :
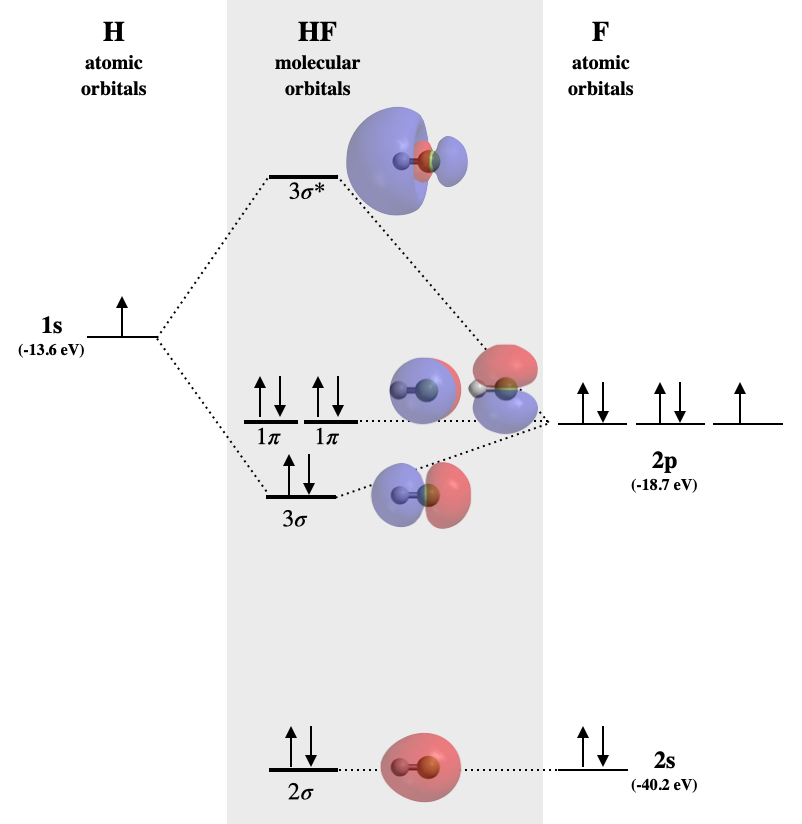
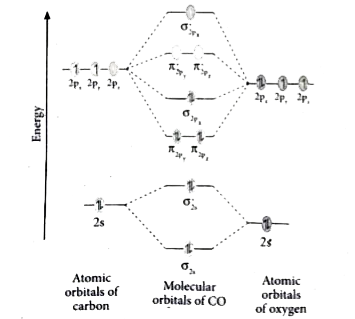
Molecular orbital diagram of Carbon monoxide molecule (CO)
Electronic configuration of C atom `1s^(2) 2s^(2) 2p^(2)`
Electronic configuration of O Draw the MO diagram for acetylide ion `C_(2)^(2-)` and calculate its bond order. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding. in molecules. The discrepancy in energies allows the π2px & π2py bonding molecular orbitals to sink lower in energy than the " σ*2s MO" in the MO diagram of CO. Only RUB 193.34/month. MO diagram for CO2 (linear triatomic). STUDY. Flashcards. The bonding MO has more of the lower energy AO, so the electrons will spend more time next to the atom with lower AOs, which is the same as the more electronegative atom. Example: CO. This diagram is based on calculations and comparison to experiments.
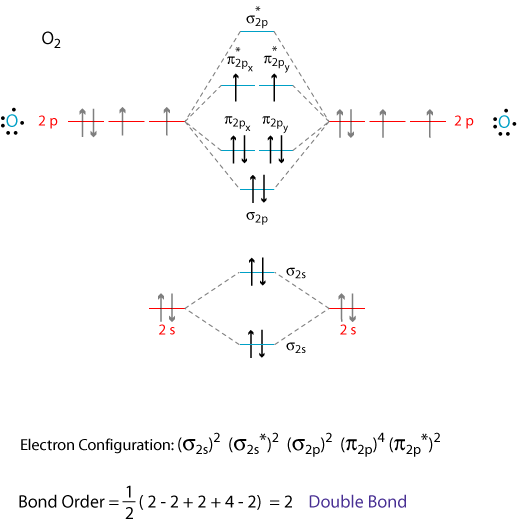
MO diagram for CO. • Same orbitals as homonuclear diatomics - different energies give rise to significant 2s - 2p mixing - confusing set of orbitals. 3σ CO. LUMO - 2π* Comes from standard π interaction however lower oxygen orbital means π has has more oxygen and π* more carbon. π π∗. Relative AO Energies for MO Diagrams. CO2 is also linear. Here all three atoms have 2s and 2p orbitals to. Construct MO diagrams for simple linear triatomic molecules and/or compounds. What's Different if we have 3 Atoms? We won't go into the details of MO theory for complex molecules, because that's a topic for more advanced classes, like Inorganic Chemistry. 22.Chemistry | Chemical Bonding | MO diagram for CO & NO Molecule. Смотреть позже. Поделиться.
Understandably, the key difference between these molecules is that $\ce{CO}$ is heteronuclear, and thus will have differences in energy between the molecular orbital and the In the above MO diagram, the 5σ is the HOMO. But it is closest in energy to oxygen's 2p orbitals, so why is it centered on carbon? Although MO theory in principle gives us a way to calculate the energies and wavefunctions of For example, you should have no trouble drawing the VB pictures for CO, NH3, and benzene, but we will Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic... Hello, r/Chemistry! I've been suddenly thrust into teaching an undergrad inorganic course because the original instructor unfortunately had to take emergency medical leave. It looks like I'll be at the helm for the remainder of semester. The problem is that the book he chose for the course is downright *awful.* (I think he picked it only because it was available as an e-book for free to the students through our university library system.) I'd hate to ask students to purchase a new book halfway ... MO diagram A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of Dihelium (He-He) is a hypothetical molecule and MO theory helps to explain why. The MO diagram for dihelium (2 electrons in each 1s AO) looks...
of the other ligands on the metal, the lower the CO stretching frequency. 3. For simple carbonyl complexes, counting the number of IR and Raman CO stretching frequencies will often permit one to make a structural assignment. The number of CO stretches expected for possible geometries/isomers...
The Unified Modeling Language (UML) is a general-purpose, developmental, modeling language in the field of software engineering that is intended to provide a standard way to visualize the design of a system.. The creation of UML was originally motivated by the desire to standardize the disparate notational systems and approaches to software design.
Thank you for recommendations.
Figure 1. MO diagram of F2. Likewise, the 2p atomic orbitals will combine to form bonding and anti-bonding interactions. Like the 2s atomic orbitals Figure 2. Linear combination of ligand atomic orbitals of [Co(NH3)6]Cl3. To generate the SALCs for [Co(NH3)6]3+, we follow a similar procedure outlined in...
MO Diagram for the Ammonia Molecule. 5.03 Inorganic Chemistry. Accurate MO calculations provide the total electron density and predict observable properties (vibrations, NMR, electronic transitions, magnetism) MOs have the symmetry of the irreducible representations maximizing...
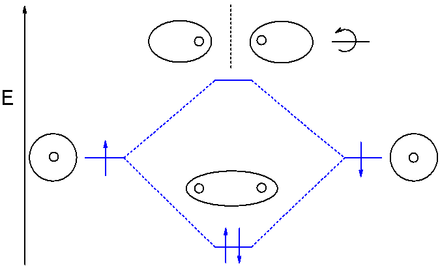
I get the meaning of MO for diatomic molecules such as O2 F2 and similar, but i'm stuck on how to know which orbitals have more energy and how to identify anti-bonding ones. I searched on internet but it's not quite helpful
Mar 31, 2015 · The MO diagram for generic metallocenes, Cp 2 M is shown below. Notice that the Cp orbitals fill the six lowest orbitals. The next five unoccupied MO's shown in the box have little or no bonding character, which explains our observation above that metallocenes are known for a variety of d-electron counts.
CHANNEL RUSTIC LAP siding pattern diagram and customer pictures of cedar siding or redwood siding. Custom milled and delivered to your jobsite. Western Red cedar Channel Lap siding WWPA patterns and California Redwood Channel Lap patterns. National Delivery and International Delivery
Dear community, could someone explain to me, why Xe only puts 1 p-orbital into the bond? Where are the other 2? [This picture](https://i.stack.imgur.com/4Wuhw.jpg) shows really well, what I mean. In the above case of NO, the N puts in 3 p-orbitals with 1 electron each and O puts in 3 with 2-1-1. This leads to 6 MOs, 3 of whch are bonding and 3 of which are anti-bonding. Now beneath, it shows XeF2. Shouldn't both F bring in 3 p-Orbitals each with 2-2-1 and Xe 3 p-orbitals with 2-2-2? An...
I am doing an exercise about the MO diagram for SF4. For sulfur, the 2s orbital and one of the P orbitals have the A1 Mulliken symbol, and 2 of my SALCs for F have the A1 Mulliken symbol as well. I know that I will get one bonding and one antibonding MO from their interaction, but would the other two A1 MOs be nonbonding, or would one be bonding and one antibonding? If I make it so out of my four A1 MOs, two are bonding and two are antibonding, I get a bond order of 4, which makes sense for...
Symmetry and MO Diagrams for the first row Hydrides AHn The Use of Symmetry in Polyatomic Molecules With polyatomic systems, there An MO description is best developed by first considering CO2, which has the same symmetry analysis (except that XeF2 brings np valence AOs as well as the...
Dear community, could someone explain to me, why Xe only puts 1 p-orbital into the bond? Where are the other 2? [This picture](https://i.stack.imgur.com/4Wuhw.jpg) shows really well, what I mean. In the above case of NO, the N puts in 3 p-orbitals with 1 electron each and O puts in 3 with 2-1-1. This leads to 6 MOs, 3 of whch are bonding and 3 of which are anti-bonding. Now beneath, it shows XeF2. Shouldn't both F bring in 3 p-Orbitals each with 2-2-1 and Xe 3 p-orbitals with 2-2-2? And...
Determine the following for CO32−. C-O bond order. the hybridization of the carbon atom. • Use an MO diagram to predict the number of unpaired electrons in a molecule. • Explain how to determine the relative phases of the atomic orbitals used to construct the molecular orbitals for molecules with more...
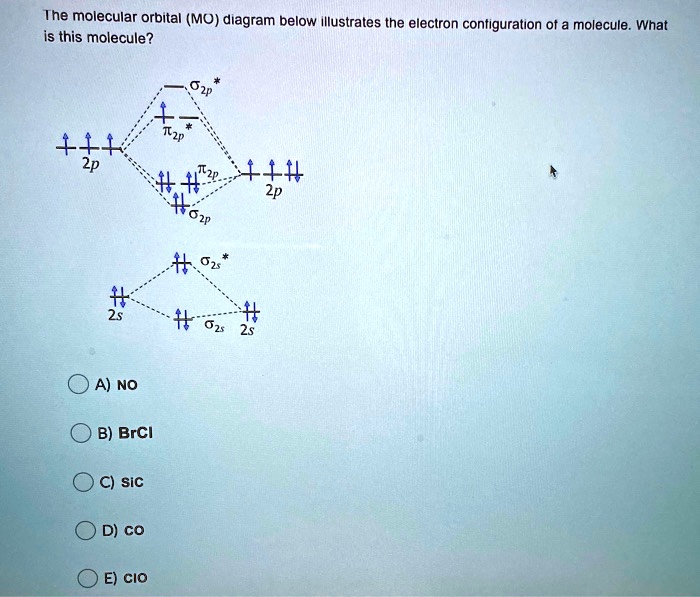
Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the `2s` orbitals and 1 electron in the `2p` orbital. That's it for the MO With MO diagrams, we can predict the number of bonds in diatomic molecules. For example, here's the MO diagram for `N_2`. We know from the...
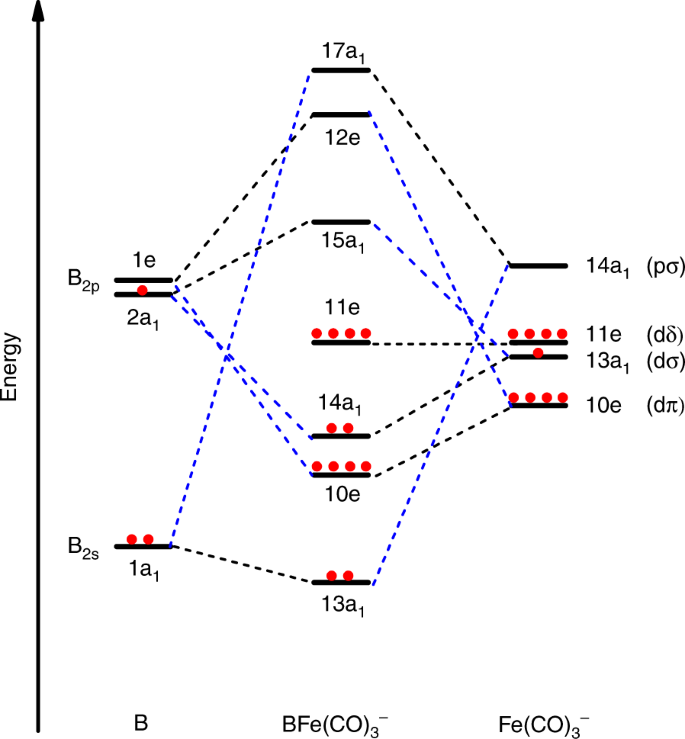
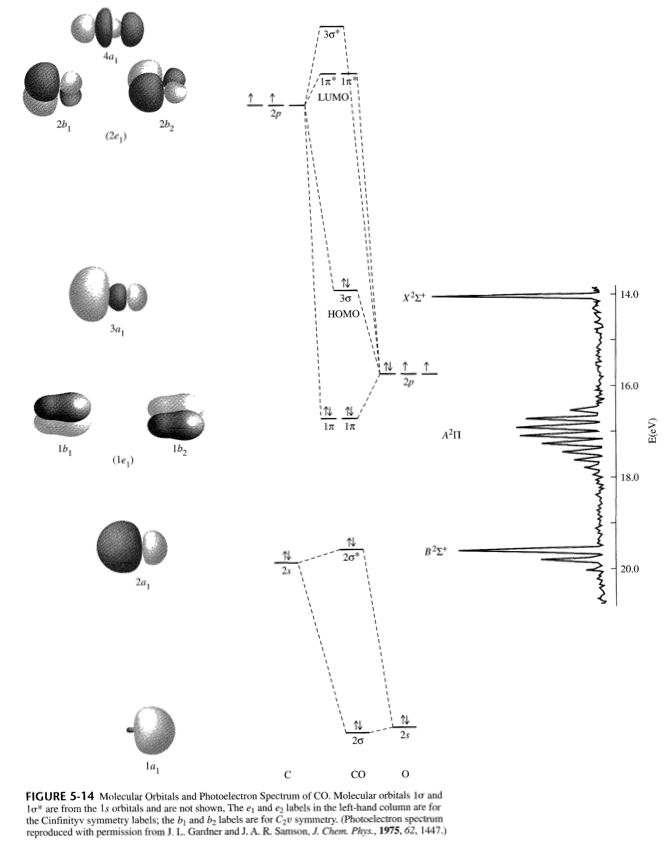
Draw a complete MO diagram for CO, marking the energies of the various occupied MOs on your diagram. In MO 7 of CO most of the electron density is localised on carbon, making this orbital effectively non-bonding, and leading to its approximate description as a carbon lone pair.
where is the lattice co-ordination number.This can be obtained from the full Bose-Hubbard Hamiltonian by setting ^ → + ^ where = ^ , neglecting terms quadratic in ^ (which we assume to be infinitesimal) and relabelling ^ → ^.Because this decoupling breaks the () symmetry of the initial Hamiltonian for all non-zero values of , this parameter acts as a superfluid order parameter.
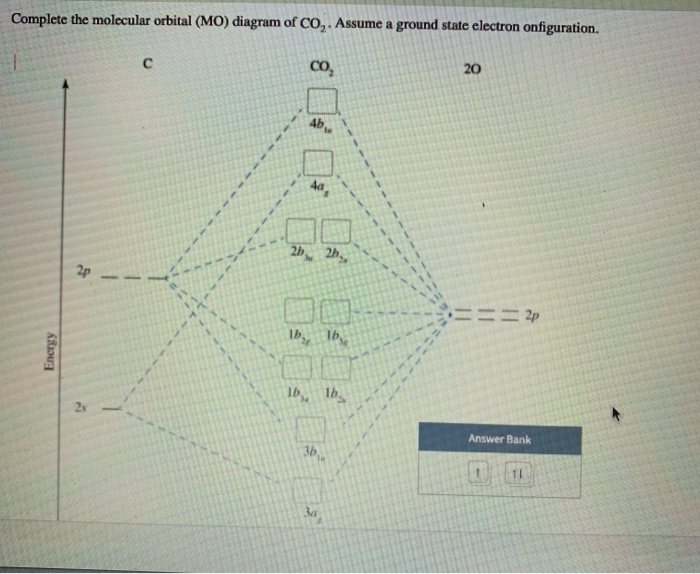
The molar mass of CO2 is 44.009 g/mol and density is 1562 Kg/m3. Now let us learn the basic concepts about CO2 molecules. CO2 Lewis Structure. All the 16 electrons are filled precisely as per rules. The antibonding orbitals are vacant in the case of CO2 as observed from the MO diagram.
Just judging from an accurate MO diagram is sufficient, here I'd say the bonding orbitals are 1 sigma and 2 x 1 pi whilst the remaining occupied sigma For example, if the was higher in energy than the then there would be two unpaired electrons and CO would show paramagnetism (which it doesn't).
Let us help you. Give us a call or send us an email. Our dedicated staff is your premier source for educated sales, design and after-sale support.
Draw the MO diagram for CO (carbon monoxide). What is the bond order of this molecule?
Mo Diagram For Co. Posted on April 6, 2019April 6, 2019. Sponsored links. Related posts: Ao Smith Motor Wiring Diagram. Ford F350 Wiring Diagram For Trailer Plug. Diagram Of A Boat. Delta Modulation Circuit Diagram.
File:MO Diagram CO2.jpg. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. File:MO Diagram CO2.svg is a vector version of this file. It should be used in place of this JPG file when not inferior.
Hello everyone, I am teaching MO diagrams next week and would like to make a proper Figure for the corrections of my exercises. Since I am no Picasso, doing it by hand seems tedious for the students. How do you do that guys? I am looking at doing it with Tikz but since I am doing BF3, that gets messy quite fast. Python perhaps? I would be thankful if someone had advices or a working script, bonus cookie points if it is on one of the molecules I will make the students suffer on. Cheer...
... simple molecular orbital (MO) diagram for CO is shown below (Figure 1). The highest occupied molecular orbital (HOMO) is indicated by the pair of ... et al. studied cyclopentadienylmolybdenum carbonyls Cp2Mo2(CO)n (Cp = η 5 -C5H5; n = 6−1) (Figure 12) by density functional theory and...
Does it mean that CO should have a double bond instead of a triple bond? (but then octet rule will not apply due to limited amount of valence electrons) or is this an exception where MO diagram and lewis structure are not congruent?
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
2. Electronic configuration of CO molecule is Draw the MO diagram for acetylide ion C2^2- and calculate its bond order.
In the MO diagram for CO 2 in problem #36 , the stretched O 2 molecular orbitals have very little energy difference ( E). Why is the energy difference between the MOs in stretched O much smaller than the energy difference between the same MOs in non-stretched O 2 ? 40. For each species below...











![Exchange coupling through diamagnetic [Fe(CO) 4 ] 2 ...](https://pubs.rsc.org/image/article/2011/DT/c0dt01221a/c0dt01221a-f2.gif)




0 Response to "37 mo diagram for co"
Post a Comment