38 draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
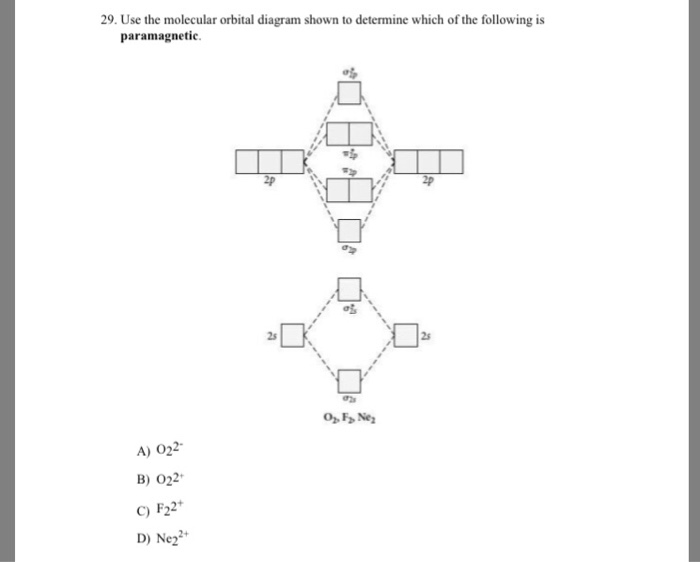
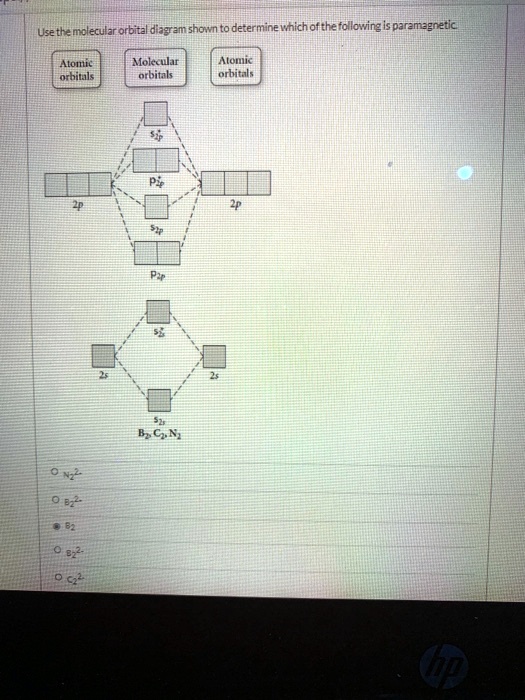
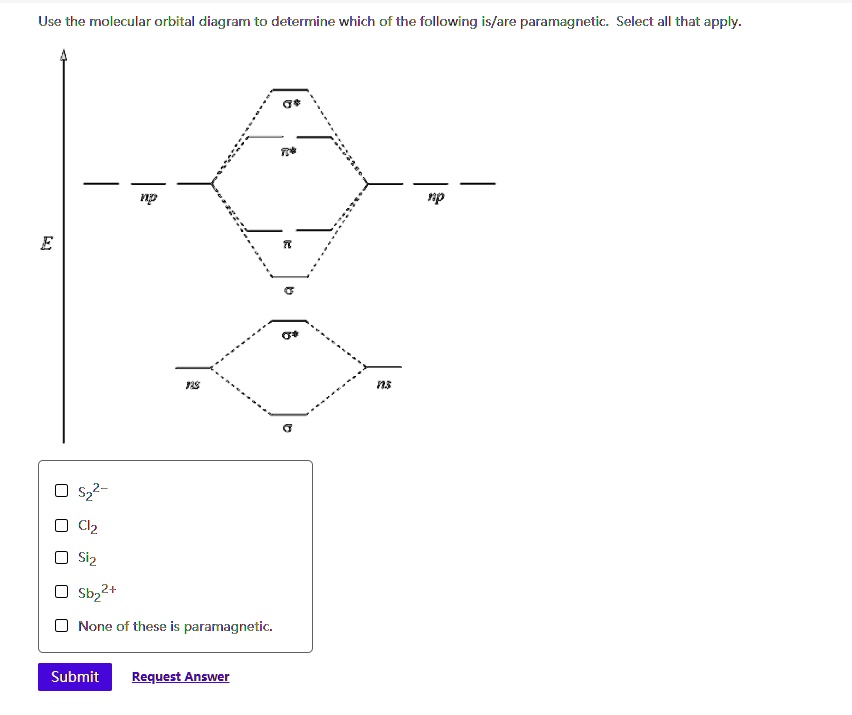
Draw a molecular orbital diagram and use it to determine which of the following is paramagnetic. F22+ Ne22+ O22- O22+ None of the above is paramagnetic. 59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O 2 2 ⁻ B) Ne 2 2 ⁺ C) O 2 2 ⁺ D) F 2 2 ⁺ E) None of the above are paramagnetic. Answer: D
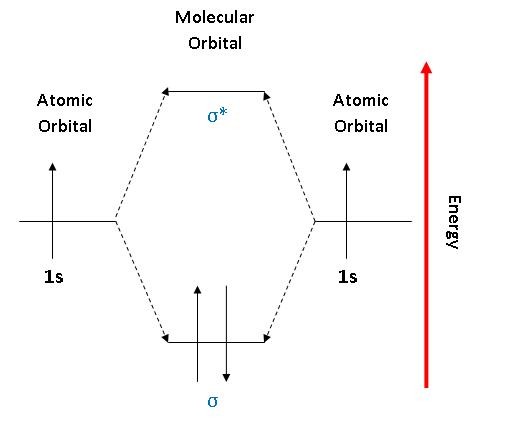
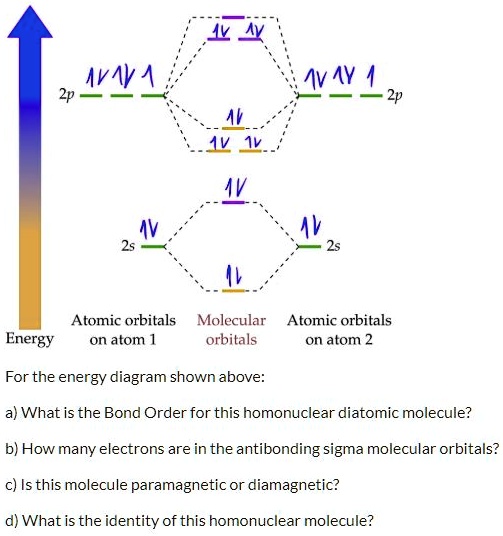
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
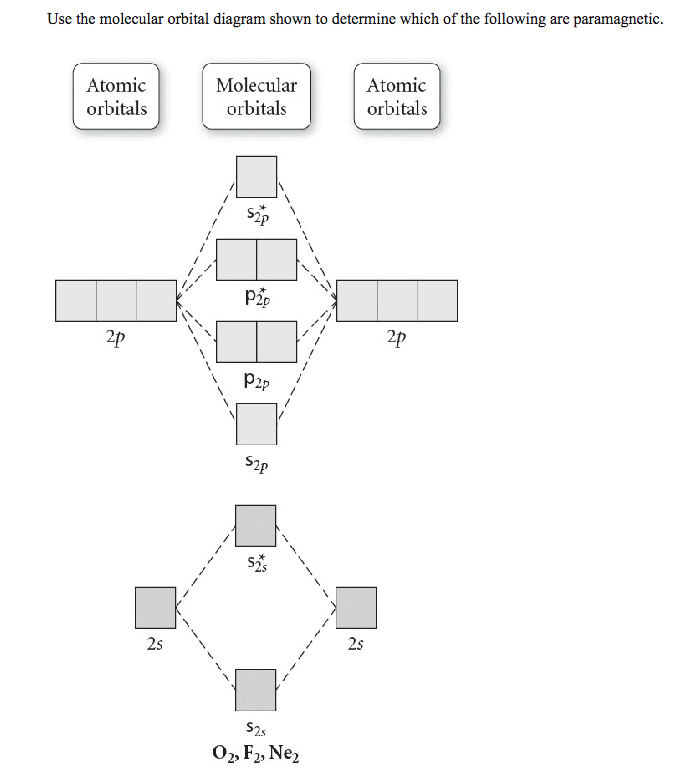
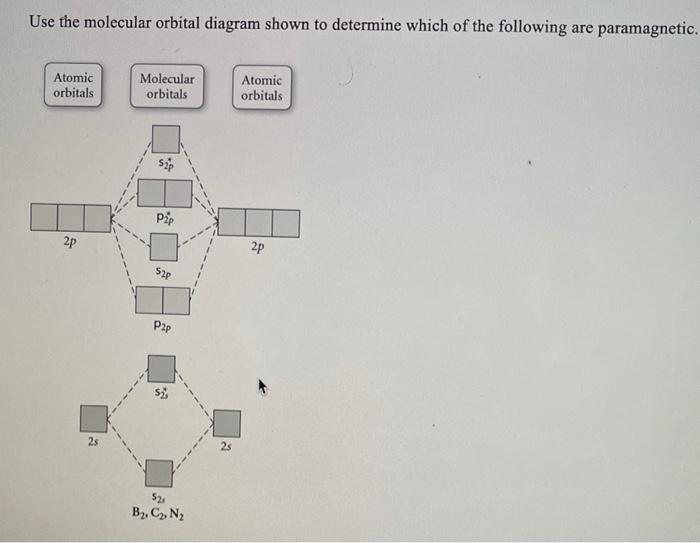
Chemistry questions and answers. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2p 2P קני 25 02 02, F2 Nez F2 None of the ions are paramagnetic. 2+ 02 2-. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. F22+. O22-. Ne22+. O22+. None of the above are paramagnetic. Best Answer. This is the best answer based on feedback and ratings. 100% (46 ratings) FREE Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.. Use the molecular orbital diagram shown to determine which of the following are paramagnetic. A. Ne 22+ B. O 22+ C. F 22+ D. O 22- E. None of the above are paramagnetic. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos. Academia.edu is a platform for academics to share research papers. In these diatomic molecules, several types of molecular orbitals occur. ... the MO diagrams shown in Figure 11, we can add in the electrons and determine ... Use the molecular orbital diagram shown to determine which of the following is paramagnetic. a) B2 b) C2^2- c) N2^2+ d) B2^2- e) B2^2+ Use the molecular ...
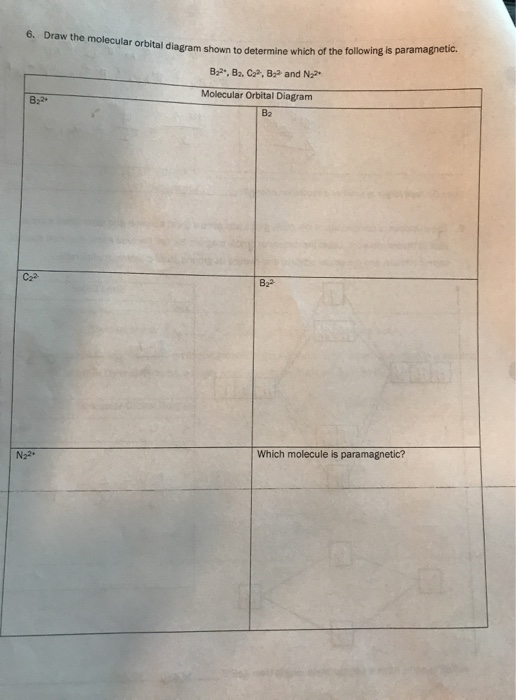
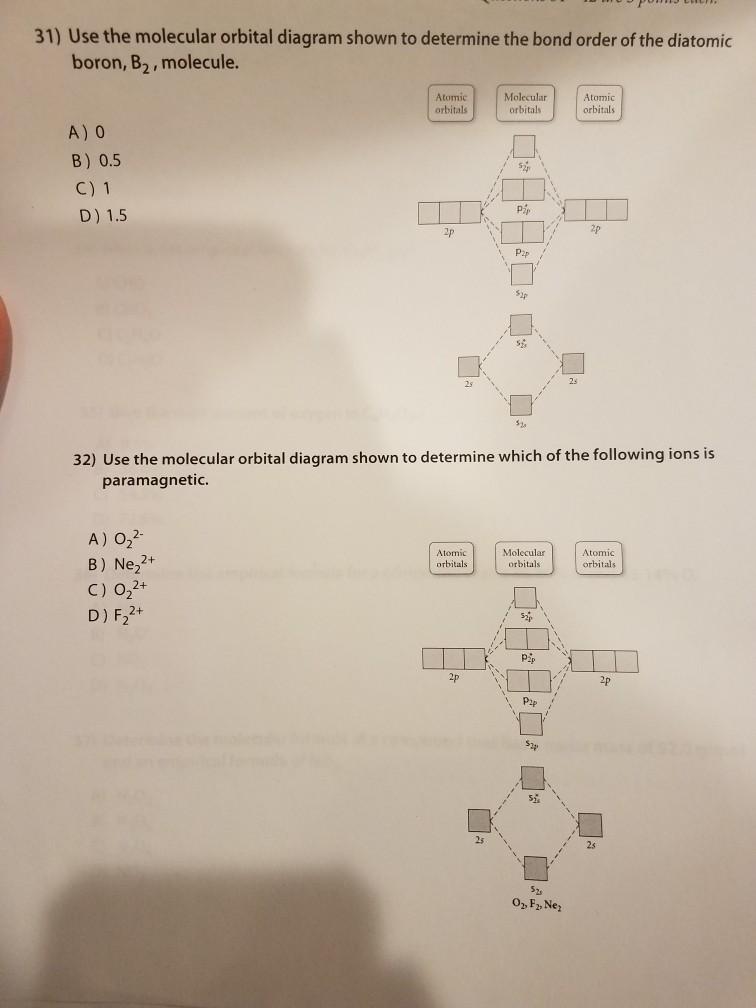
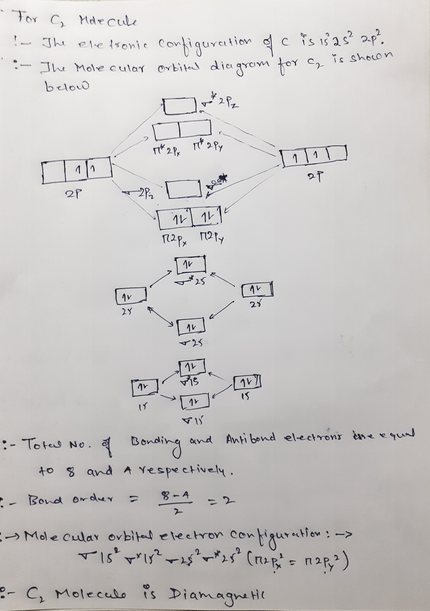
The molecular orbital diagrams for molecules and ions are drawn from the order of increasing energies shown in the molecular orbital configuration. Always remember that the number of molecular orbitals formed must be equal to the number of atomic orbitals that were combined in the molecule. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+. B22+. B22-. B2. C22-. Solution.pdf. Sign In to Writing (Essays) Science. Chemistry Q&A Library Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B22+, B2, C22-, B22-, and N22+. Start your trial now! #1. Draw the MO diagram for `B_2`. First step is to determine which MO diagram we're using. In this case, we're using the standard one. Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the `2s` orbitals and 1 electron in the `2p` orbital. That's it for the MO diagram of `B_2`! To check, count how many ...
Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy ... We need to look at the molecular orbital (MO) diagram for each molecule; to do so: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*). 2. The bond order of a homonuclear diatomic molecule can be decreased by. removing electrons from a bonding MO or adding electrons to an antibonding MO. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) N2^2+. B) B2^2+. C) B2^2-. D) C2^2-. E) B2. Use the molecular orbital diagram shown to determine which of the following are paramagnetic. B₂ Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Use the molecular orbital diagram shown to determine which of the following are paramagnetic Atomic orbitals Molecular orbitals Atomic orbitals pã µ 2p 2p ...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. Answer options: Ne22+ O22- F22+ O22+ None of the above are ...
Draw the molecular orbital diagram to determine which of the following is paramagnetic. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Nitrogen can lose an electron to form N2+.

Draw the appropriate molecular orbital diagram and determine which of the following are paramagnetic
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.Answer options:B2B22+N22+C22-B22-
use the molecular orbital diagram shown to determine which of the following are paramagnetic. F2 ^2+ how many of the following ionic compounds are insoluble in water? NaCl KI Mg(NO3)2 CaCO3 PbI2. 2. give the theoretical yield, in moles, of CO2 from the reaction of 4.00 moles of C8H18 with 4.00 moles of O2
a. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. B_2^2+, B2, C_2^2-, B_2^2- and N_2^2+ b. Draw the Lewis structures and molecular orbital diagrams ...
Science. Chemistry. Chemistry questions and answers. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. O 0,2 O F₂2+ O Ne 2+ O 0₂2+ None of the above are paramagnetic. Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
Transcribed image text: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. (a) O^2-_2 (b) Ne^2+_2 (c) O^2-_2 (d) ...
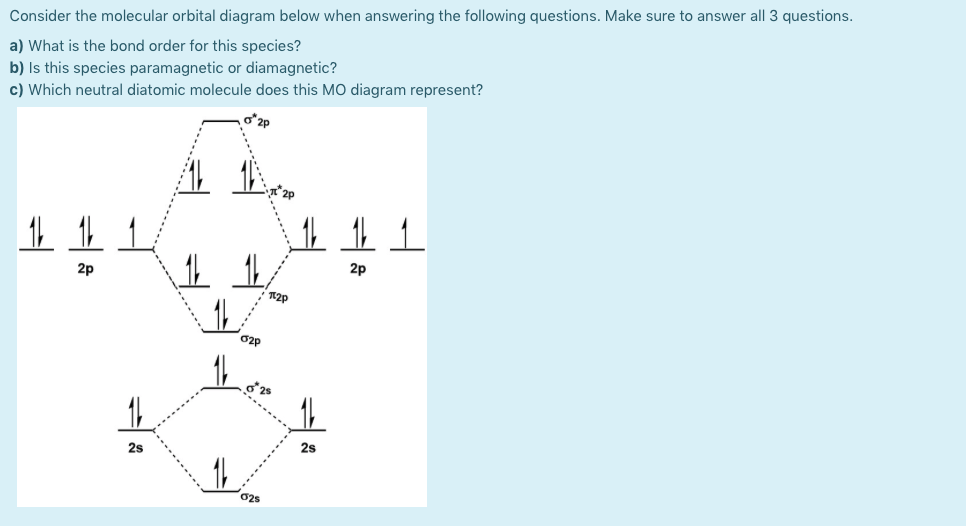
Problem: Use the molecular orbital diagram shown to determine which of the following is most stable.a.F22+b. Ne22+c. F22-d. O22+e. F2
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular…
E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+ B) N2^2+ C) B2
24.11.2021 · Two-dimensional materials (2DMs) have attracted tremendous research interest over the last two decades. Their unique optical, electronic, thermal, and mechanical properties make 2DMs key building blocks for the fabrication of novel complementary metal–oxide–semiconductor (CMOS) and beyond-CMOS devices. Major advances in device functionality and performance …
18 Full PDFs related to this paper. READ PAPER. solucionario quimica de raymond chang 12 edicion
Use the molecular orbital diagram shown to determine which of the following paramagnetic. Ne_2^2+ O_2^2+ F_2^2+ O_2^2- None of the above are paramagnetic.
Construct the molecular orbital diagram of NO+ and calculate for the bond order and determine if its paramagnetic or diamagnetic. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Determine if there's an unpaired MO (paramagnetic or diamagnetic)
Transcribed image text: 6. Draw the molecular orbital diagram shown to determine which of the following is paramagnetic B,2 B, C2, B22 and N22.
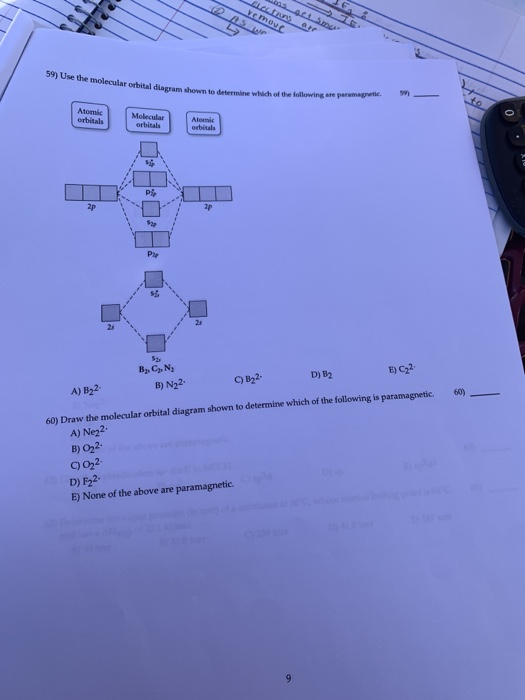
59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+. 60) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+.
Draw the best Lewis structure for BrO4⁻ and determine the formal charge on bromine. A) -1 B) +1 ... Draw the molecular orbital diagram shown to determine which of the following is most stable. A) F2 B) F22⁺ ... Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O22⁻ ...
Question: Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. O22+ Ne22+ F22+ O22- ...
FREE Answer to Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. N22+ B22+...
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. F22+. O22-. Ne22+. O22+. None of the above are paramagnetic. Best Answer. This is the best answer based on feedback and ratings. 100% (46 ratings)
Chemistry questions and answers. Use the molecular orbital diagram shown to determine which of the following is paramagnetic. Atomic orbitals Molecular orbitals Atomic orbitals 2p 2P קני 25 02 02, F2 Nez F2 None of the ions are paramagnetic. 2+ 02 2-.









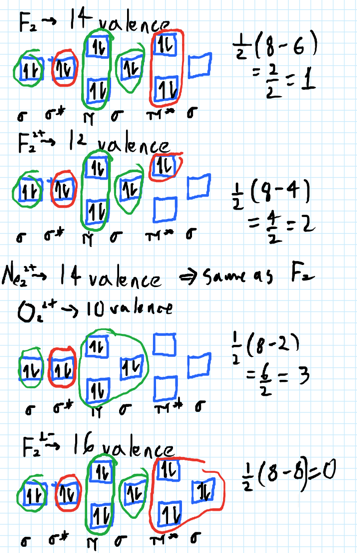






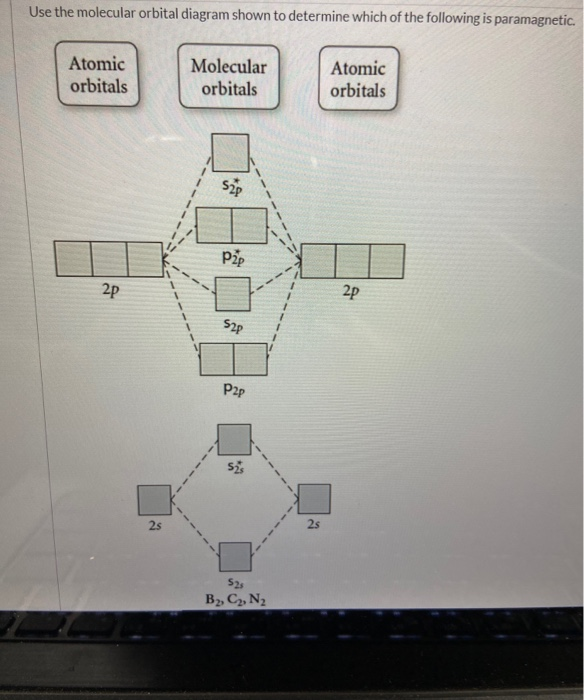

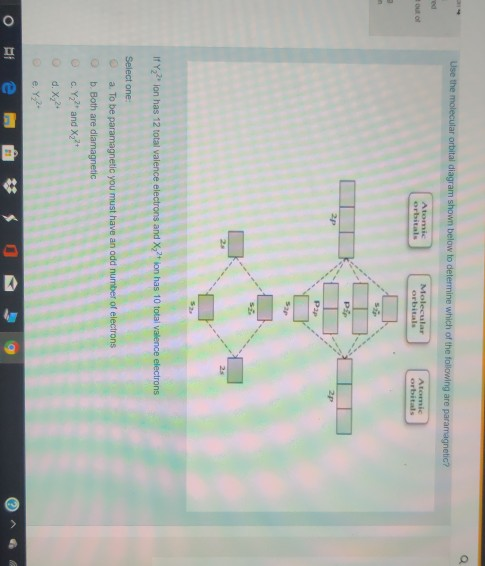
0 Response to "38 draw the molecular orbital diagram shown to determine which of the following is paramagnetic."
Post a Comment