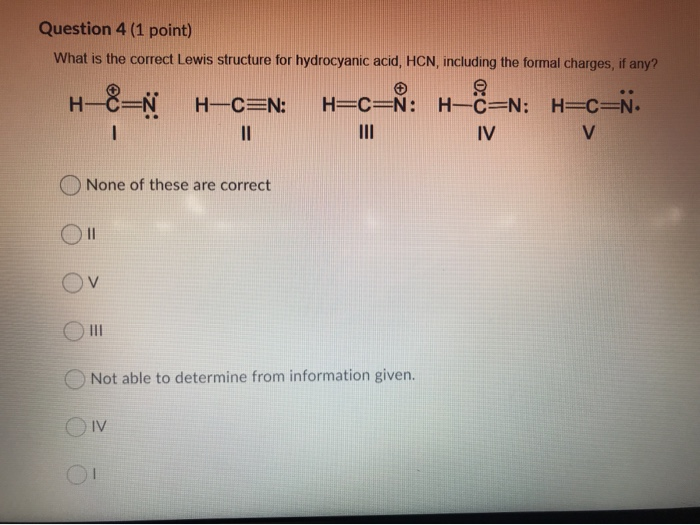
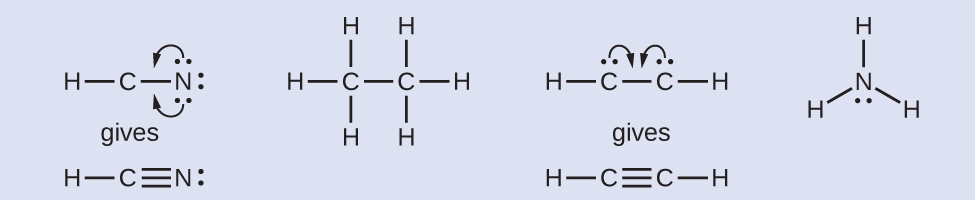
42 lewis diagram for hcn
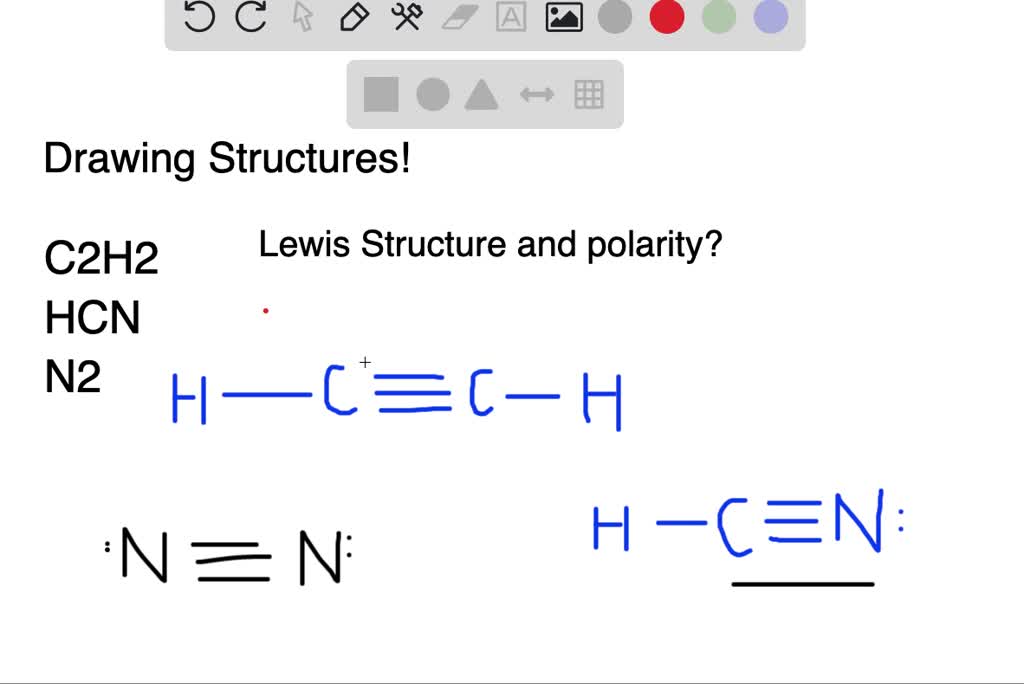
A Lewis structure with placeholder central atom is shown below. ... Draw the Lewis structure of HCN and then determine its electron domain and molecular geometries. image 20 ... Draw the MO diagram for each molecule and think about the number of bonding and antibonding electrons. hcn lewis diagram cyanide hydrogen carbon structure electrons bond pair triple atoms atom fairly otherwise known simple
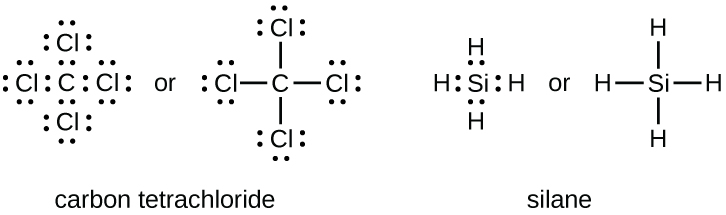
Covalent compounds lewis dot structure worksheet. One such compound is pf 5. Covalent bonding in f2 two fluorine atoms each with 7 valence electrons can share those electrons in a covalent bond. For the following molecules or ions where the central atom is underlined. Lewis structure displaying top 8 worksheets found for this concept.
Lewis diagram for hcn
26-06-2017 · Uniform circular motion, but the satellite is accelerated towards the centre of the earth. There are a few concepts with regards to a satellite orbiting the earth. The orbit is actually elliptical, but it is treated as circular for easier calculations. The satellite is orbiting with constant speed. The satellite's velocity is always changing. Lewis Diagram and Molecular Shape of Sulfur tetrafluoride SF4 Lewis Diagram and Molecular Shape of Silicon Tetrafluoride SiF4 Lewis Diagram and Molecular Shape of Nitrogen trifluoride NF3 Jul 23, 2021 · To start with making the Lewis Structure of HCN, we will first determine the central atom. And then place the remaining atoms in the structure. As Carbon is the least electronegative atom in this molecule, it will take the central position. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms.
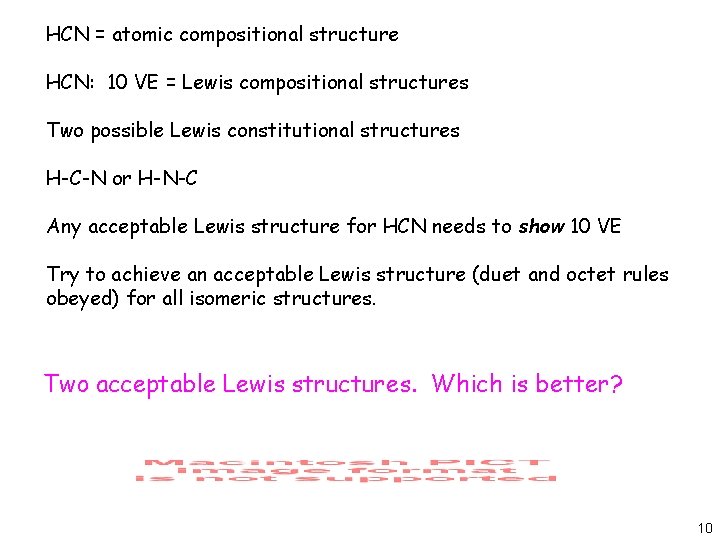
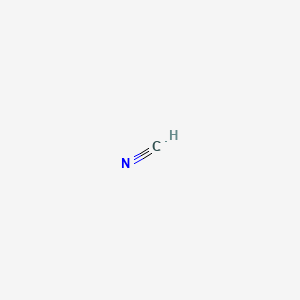
Lewis diagram for hcn. The Lewis Structure (Lewis Dot Diagram) for HCN.1. Count electrons2. Put least electronegative atom in centre3. Put one electron pair in each bond4. Fill out... Although HCN is a weak acid and normally not considered corrosive, it has a corrosive effect under two special conditions: (1) water solutions of HCN cause transcrystalline stress-cracking of carbon steels under stress even at room temperature and in dilute solution; (2) water solutions of HCN containing sulfuric acid as a stabilizer severely corrode steel above 40 °C and stainless … Lewis Structure: Bonding: Shape: 1. CH 4: 4 bonds. 0 lone pairs: tetrahedral: 2. NH 3: 3 bonds. 1 lone pair: trigonal pyramidal: 3. H 2 O 2 bonds. 2 lone pairs: bent: 4. H 3 O + 3 bonds. 1 lone pair: trigonal pyramidal: 5. HCN : 2 bonds. 0 lone pairs: linear: 6. CO 2: 2 bonds. 0 lone pairs: linear: 7. CCl 4: 4 bonds. 0 lone pairs: tetrahedral: 8. COCl 2: 3 bonds. 0 lone pairs: trigonal planar: 9. O 3: 2 bonds Hydrogen Cyanide (HCN) is a colourless, flammable, and poisonous liquid. HCN Lewis structure comprises three different atoms: Hydrogen, carbon, and nitrogen. It is a polar molecule with bond angles of 180 degrees. HCN is used in electroplating, mining, and as a precursor for several compounds. Name of molecule.
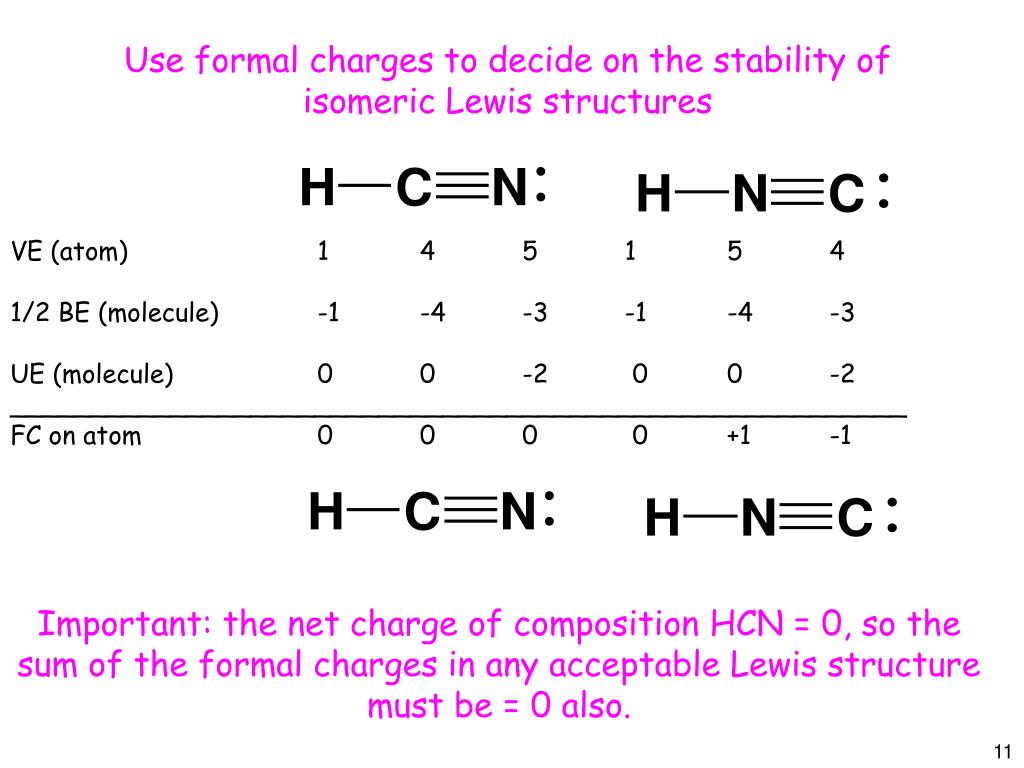
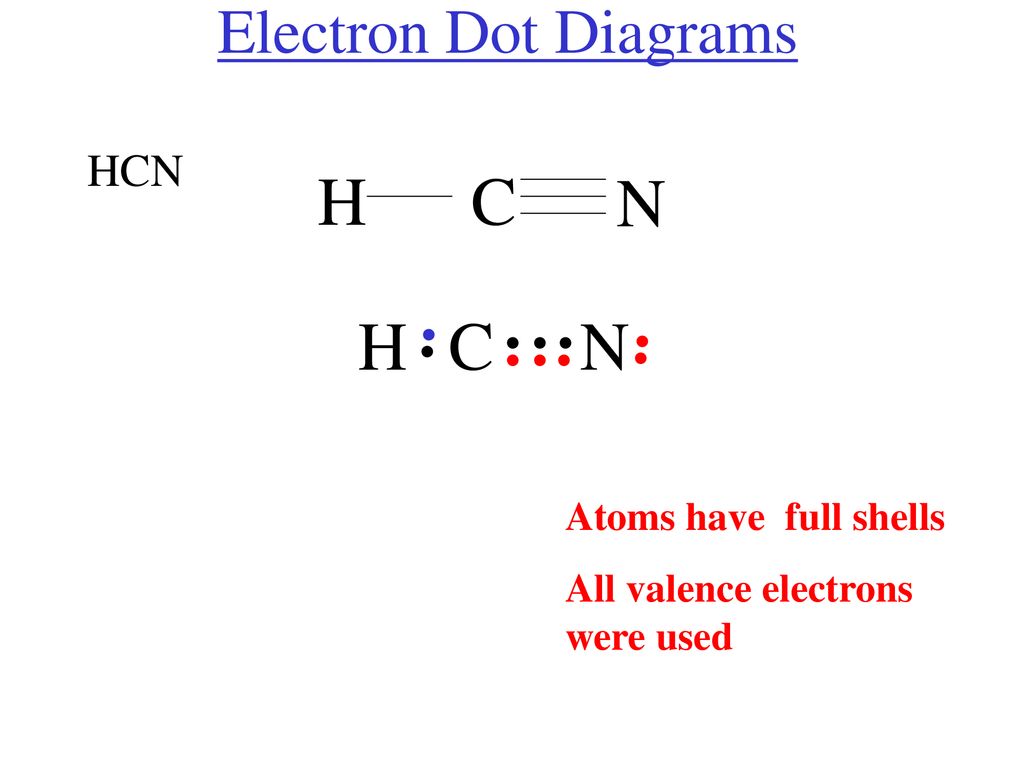
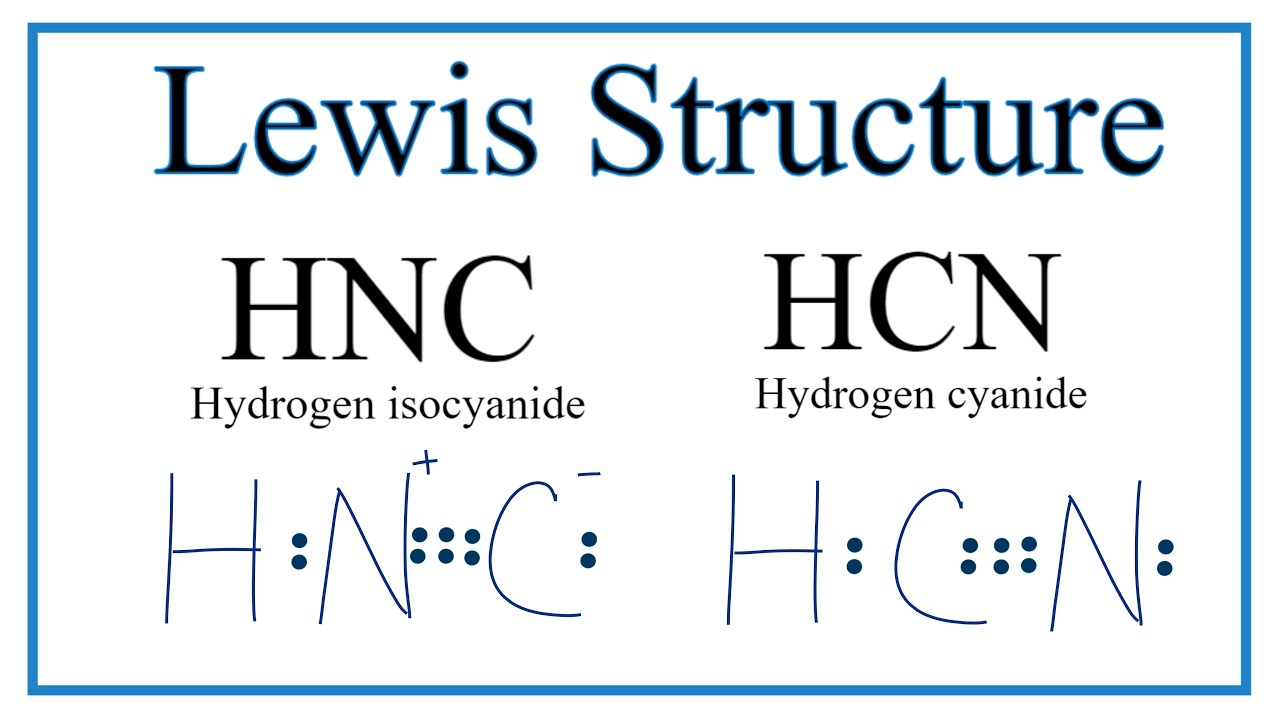
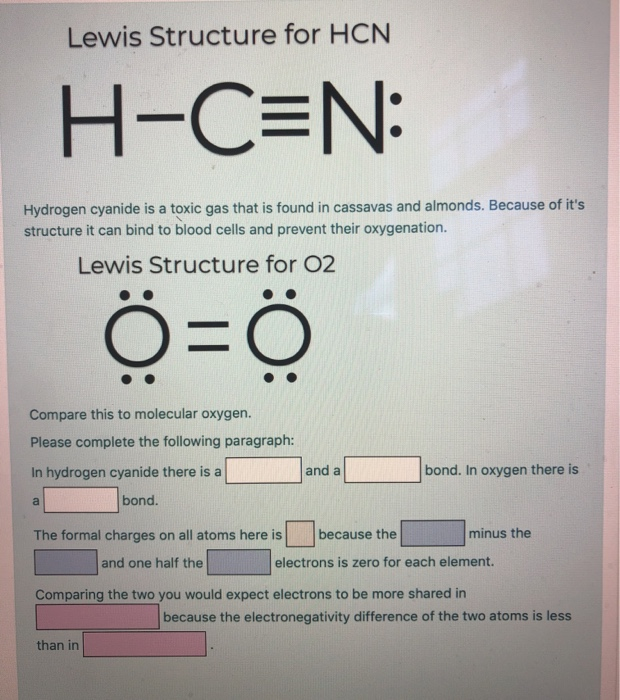
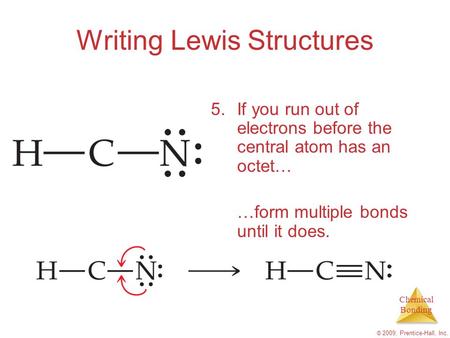
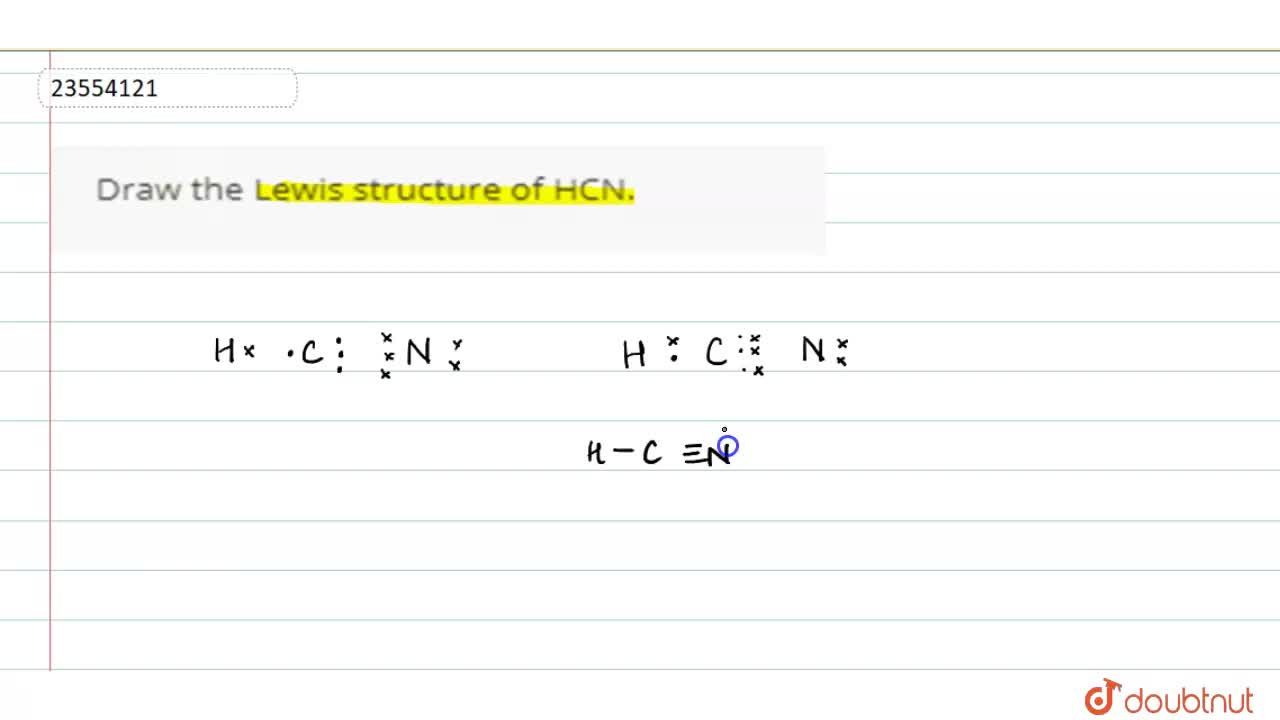
The Lewis dot diagram for hydrogen cyanide, #"HCN"#, is #"H:C:::N:"#.There is a single covalent bond between the hydrogen and carbon atom, represented by two dots, #:#, each of which represents a shared electron; a triple covalent bond between the carbon and nitrogen atom, represented by three pairs of dots, #:::#, representing three pairs of shared electrons, and a lone pair of electrons on ... 2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii. For the HCN Lewis structure, calculate the total number of valence electrons for the HCN molecule. After determining how many valence electrons there are ... 10-02-2021 · Also, hydrogen cyanide (HCN) predominates at low and middle pH values and is about twice as toxic as CN-, which is found in appreciable amounts above pH 8.5 (Rand 1995). Ammonia : Concentration and toxicity of NH 3 increases as pH increases, although less NH 3 is required to produce toxic effects at lower pH (IPCS 1986, Wurts 2003).
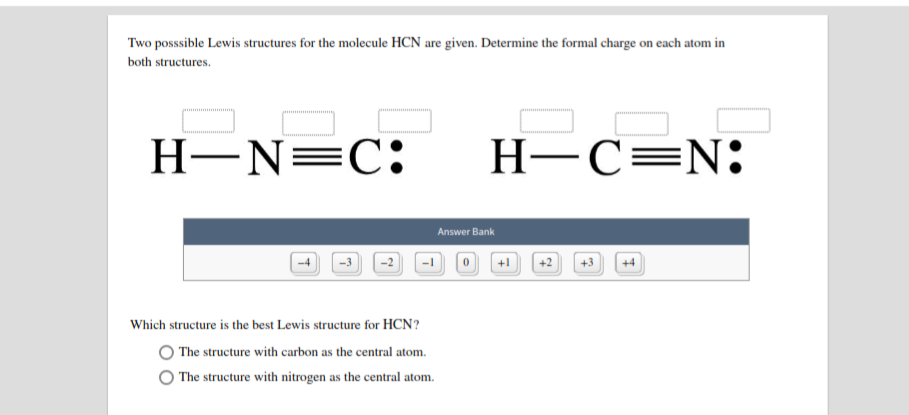
HCN has a hydrogen atom single-bonded to a carbon atom, and that carbon atom is triple-bonded to a nitrogen atom. These are all non-metals, so the bonds are covalent and HCN is therefore a covalent (aka Molecular) structure. Carbon brings four valence electrons with it; it needs four more to complete its valence shell. Stevens LA, Nolin TD, Richardson MM, Feldman HI, Lewis JB, Rodby R, et al. Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009; 54:33–42. [PMC free article] [Google Scholar] Nitrogen trifluoride (NF3) lewis structure contains three N-F bonds. 3. Mar 05, 2017 · Re: BF3 and NF3. Problem: The hybridizations of nitrogen in NF3 and NH3 are _____ and _____, respectively. Also there is one lone pair on nitrogen atom and three lone pairs on fluorine atoms. We're going to do the Lewis structure for NF3, nitrogen trifluoride. Draw the lewis dot structure for each of the following polyatomic ions. Determine if the compound is covalent or ionic. No 3 d. The only reasonable lewis electron dot diagram for this compound has the p atom making five covalent bonds. Some of the worksheets for this concept are lewis structure work practice problems h s so ch br hcn lewis ...
Drawing the Lewis Structure for HCN Viewing Notes: Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you’ll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. Be sure that you don't use more than the ten valence electrons available.
The lungs Quiz States of matter Quiz Chromatography Quiz GCSE Biology sample animations and quizzes GCSE Chemistry sample animations and quizzes GCSE Physics sample animations and quizzes GCSE Investigative Skills animations/slides
It is represented by dots in the HCN Lewis diagram. The HCN molecule's core carbon atom can be represented as follows: Total outermost valence shell electron of carbon atom in HCN= 4 Total outermost valence shell electron of nitrogen atom in HCN= 5 Total outermost valence shell electron of hydrogen atom in HCN= 1
The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond the . Sep 10, · HCN should be written H-CN, with a triple bond between C and N. Observe that the ion CN (-) is isoelectronic with the molecule of CO, carbon monoxide.
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity. Hydrogen Cyanide is a very toxic acid and is famous for causing irritation in the eyes and respiratory system if any human inhales HCN in substantial quantity. The compound is a colorless substance that is available in liquid or gaseous form.
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide.This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, H 2 CrO 4 of which the trioxide is the anhydride.
18-03-2018 · Also see here... Bond order for "NO"^+ Order by bond length: "NO", "NO"^(+), "NO"^(-) Is "CO" a Lewis acid? "O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The …
An energy diagram for the single-step bimolecular E2 mechanism is shown on the right. We should be aware that the E2 transition state is less well defined than is that of S N 2 reactions. More bonds are being broken and formed, with the possibility of a continuum of states in which the extent of C–H and C–X bond-breaking and C=C bond-making varies.
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
Hcn bond angles of 180 degrees. Hydrogen cyanide is a linear molecule with a triple bond between carbon and nitrogen. Simple Procedure for writing Lewis Structures - Lewis The lewis structure lewis dot diagram for hcn. Lewis diagram for hcn. Put least electronegative atom in centre 3. Put one electron pair in each bond 4.
Electron dot diagram HCN by Jayne Ferguson - October 14, Lewis structure example. Draw the Lewis Structure of HCN. 1. Draw the skeletal structure showing how the atoms are connected using single bonds. Usually try. An example of linear electron pair and molecular geometry is BeH2. In this example, HCN, the Lewis diagram shows carbon at the center.
So, in this article, we have learned about How to draw CN- lewis structure, its molecular orbital diagram (MO), formal charges, hybridization, and bond order. Here is a quick review of this article. The bond order of CN- is 3. CN- formal charge is -1 according to its lewis structure.
Mar 10, 2018 · H C N Lewis structure of HCN. Step method to draw lewis structure of Hydrogen cyanide. Step 1: Find valence e- for all atoms. Add them together. H C N In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs. The carbon and nitrogen are bonded through. In this example, HCN, the Lewis diagram shows carbon at the center with no lone electron pairs.
Drawing the Lewis Structure for HCN. Viewing Notes: Make sure you put the correct atom at the center of the HCN molecule. With the Lewis Structure for HCN you’ll need to share more than one pair of electrons between the Carbon and the Nitrogen atoms. The Lewis structure for HCN, otherwise known as hydrogen cyanide, is fairly simple. Place the carbon atom in the center and triple bond it to a nitrogen atom. Then bond the carbon atom to a single hydrogen atom.
Jul 23, 2021 · To start with making the Lewis Structure of HCN, we will first determine the central atom. And then place the remaining atoms in the structure. As Carbon is the least electronegative atom in this molecule, it will take the central position. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms.
Lewis Diagram and Molecular Shape of Sulfur tetrafluoride SF4 Lewis Diagram and Molecular Shape of Silicon Tetrafluoride SiF4 Lewis Diagram and Molecular Shape of Nitrogen trifluoride NF3
26-06-2017 · Uniform circular motion, but the satellite is accelerated towards the centre of the earth. There are a few concepts with regards to a satellite orbiting the earth. The orbit is actually elliptical, but it is treated as circular for easier calculations. The satellite is orbiting with constant speed. The satellite's velocity is always changing.










![CHEMISTRY TUTORIAL] Lewis Structure of Hydrogen Cyanide ...](https://images.squarespace-cdn.com/content/v1/5f02d28f35d64d2a5022eeb1/1604451040567-7LIIA50HAGVJU0SH608N/Lewis+Structures+HCN.png)









0 Response to "42 lewis diagram for hcn"
Post a Comment