41 nacl lewis dot diagram
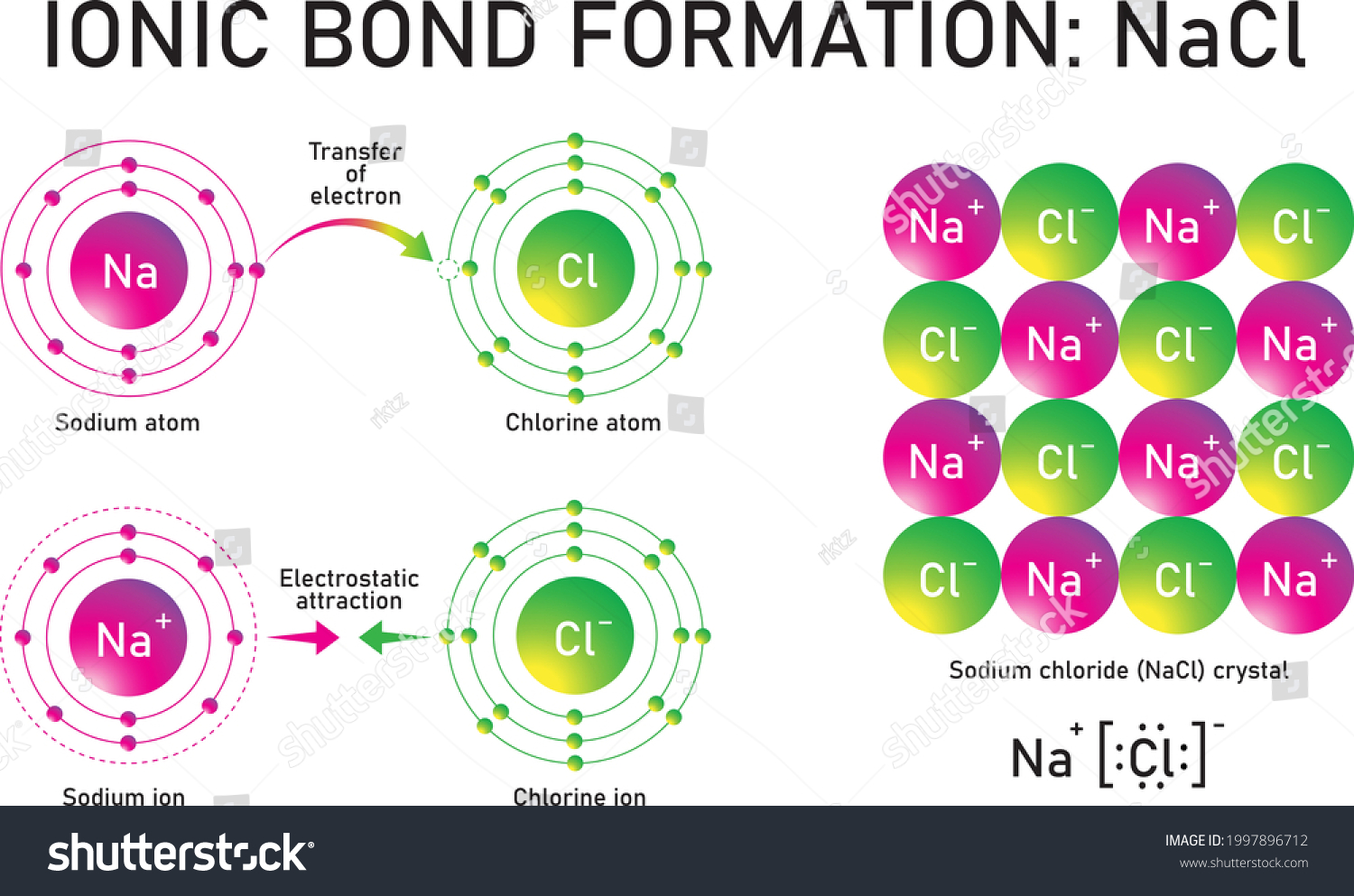
Sodium chloride (NaCl) lewis dot structure, polar or ... The lewis dot structure of NaCl contains one positive charge on sodium metal and one negative charge on chlorine nonmetal. We have to represent them by putting brackets around them. The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc. How to Draw the Lewis Structure of NaCl (sodium chloride ... Check me out:
PPTX Topic: Lewis Dot Diagrams for Ionic Compounds Topic: Lewis Dot Diagrams for Ionic Compounds. Do Now: Identify ionic compounds. CO. 2 MgCl. 2 NH. 4. ClNaOH. NH. 3 CH. 4 CuSO. 4 HF. Magnesium's outer shell is now empty. Fluorine's outer shell is now full. Lewis Diagrams for Ionic Compounds. NaCl's Lewis structure:
Nacl lewis dot diagram
PDF Lewis Dot Structures of Atoms and Ions 3. Is the reaction product (sodium chloride) more or less stable than the reactants (sodium metal and chlorine gas)? Explain. Model: Drawing Lewis Dot Structures for Atoms and Ions A. Lewis dot structure for an atom of chlorine is . The number of valence electrons for an atom is the number of electrons in the outer energy level (shell) of the atom. Draw electron dot representation for the formation of ... Draw electron dot representation for the formation of sodium chloride. Medium. ... The structure of dot representation for the formation of NaCl is written above. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > Was this answer helpful? 0. 0. Similar questions. Draw electron dot representation for the ... Multimedia: Represent Bonding with Lewis Dot Diagrams ... The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The lines are a short-hand version of the two dots representing the covalent bonds.
Nacl lewis dot diagram. NaCl Lewis dot structure? - Answers What? Lewis dot for ionic? If you want Lewis dot for say, NaCl it would be [Na]^+ [Cl^-] and the Cl^- will have eight dots around it and the Na^+ will have no dots around it. [DIAGRAM] Sodium Chloride Dot Diagram | Aruba Com Lewis Dot Diagram For The abeyant diagram aloft is for aerial NaCl, and the ambiance is altered in the accustomed solid accompaniment area sodium chloride (common table salt) forms cubical crystals. The ion break is 0.28 nm, somewhat beyond than that in the aerial state. Draw the Lewis Structure of NaCl (sodium chloride) - YouTube Sodium, a metal in Group 1, loses one electron to become a +1 ionChlorine, a non-metal in Group 17, gains one electron to become a -1 ion.Together, they comb... NATIONAL SENIOR CERTIFICATE GRADE 10 - Department of Basic ... 19/11/2018 · diagram of this ion. (3) 4.6 When orbitals of identical energy are available, electrons are placed in individual orbitals before they are paired. Give the name of this rule. (1) 4.7 Element Y occurs as these isotopes in the following proportions: Y …
How to Draw the Lewis Dot Structure for NaCl: Sodium ... A step-by-step explanation of how to draw the NaCl Lewis Dot Structure (Sodium chloride).For NaCl we have an ionic compound and we need to take that into acc... Ionic Bonding Animated! | Lewis Dot Diagram and Octet Rule ... How Ionic compounds are formed in the form of an animation!Enjoy!NOTE: The Lewis Dot Diagram in the end is the one for Sodium Chloride.Don't forget to subscr... Lewis Dot Structure of Molecules Flashcards | Quizlet In a lewis dot diagram, each element will share 8 electrons (there are exceptions like hydrogen and boron) ... electrons. structural formula lone pairs. Diagram that Shows the bonds formed between atoms as well as the lone pairs. sodium chloride (Lewis Dot Diagram. calcium carbonate. nitrogen monoxide. NO. YOU MIGHT ALSO LIKE... 18. Aviation ... NaCl Lewis Structure: How to draw the Lewis Dot Structure ... A step-by-step explanation of how to draw the NaCl Lewis Dot Structure.For the NaCl Lewis structure, calculate the total number of valence electrons for the ...
Octet rule - Wikipedia The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas.The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium. Draw the Lewis Structure of NaCl (sodium chloride) - YouTube Na (sodium), a metal, loses one electron to become a +1 ionCl (chlorine), a non-metal, gains on electron to become a -1 ion.Together, they form NaCl, one uni... PPT PowerPoint Presentation - Chemical BONDING Chemical BONDING IONIC Lewis Dot Diagrams Sodium Chloride This is the finished Lewis Dot Structure [Na]+1 [ Cl ]-1 How did we get here? Practice Dot diagrams & formulas Lithium fluoride Magnesium oxide Calcium chloride Potassium hydride Drawing molecules using Lewis Dot Structures Remember: atoms are sharing e- to complete their outer shell! Lewis Dot Diagram For Sodium Chloride - schematron.org Diagram of bonding in sodium chloride. A sodium atom gives an electron to a chlorine atom. The result is a sodium ion (2,8)+ and a chloride. In Ionic Bonds valence electrons are completely transferred (not shared). Thus, we write the Lewis structure for NaCl as: NaClLewisDot.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Exercises. 1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. 2.
How to draw NaCl Lewis Structure? - Science Education and ... It is represented by dots in the NaCl Lewis diagram. The NaCl molecule's core chlorine atom can be represented as follows: Total outermost valence shell electron of chlorine atom in NaCl= 7 Total outermost valence shell electron of sodium atom in NaCl= 1 The NaCl molecule has one central chlorine and one sodium atoms.
What Is the Lewis Dot Structure of NaCl? - Reference.com The Lewis dot structure of NaCl consists of a chloride ion surrounded by eight electron dots (four pairs) and a sodium ion bonded to that chlorine ion. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1.
Molecular Structure: Lewis Dot For example, consider sodium chloride. The Lewis Structure for the Salt NaCl, shows two ions which have their (Now) outer shells of electrons filled with a complete octet. In the case of the sodium cation, the filled shell is the outermost of the 'core' electron shells. ... Lewis dot diagrams give us a static picture of what the molecule or ion ...
Multimedia: Represent Bonding with Lewis Dot Diagrams ... The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The lines are a short-hand version of the two dots representing the covalent bonds.
Draw electron dot representation for the formation of ... Draw electron dot representation for the formation of sodium chloride. Medium. ... The structure of dot representation for the formation of NaCl is written above. Solve any question of Chemical Bonding and Molecular Structure with:-Patterns of problems > Was this answer helpful? 0. 0. Similar questions. Draw electron dot representation for the ...
PDF Lewis Dot Structures of Atoms and Ions 3. Is the reaction product (sodium chloride) more or less stable than the reactants (sodium metal and chlorine gas)? Explain. Model: Drawing Lewis Dot Structures for Atoms and Ions A. Lewis dot structure for an atom of chlorine is . The number of valence electrons for an atom is the number of electrons in the outer energy level (shell) of the atom.











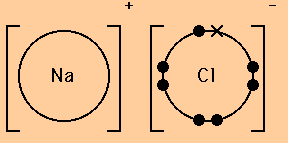




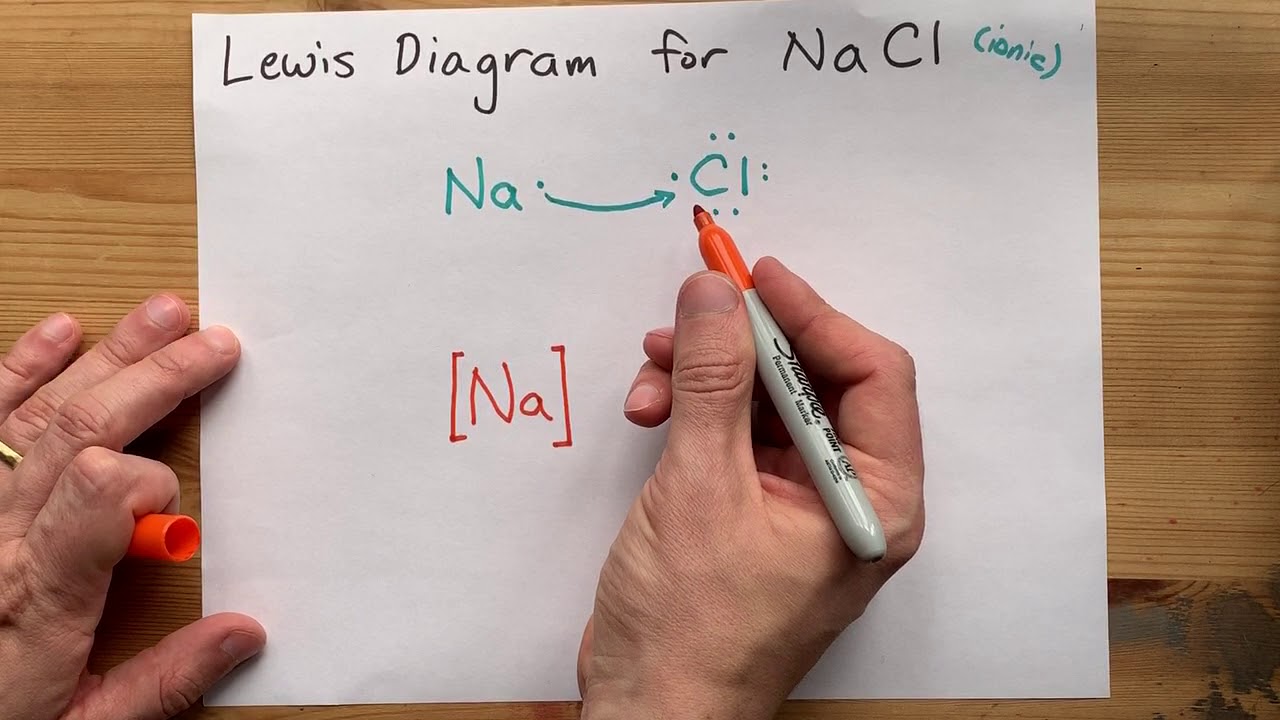



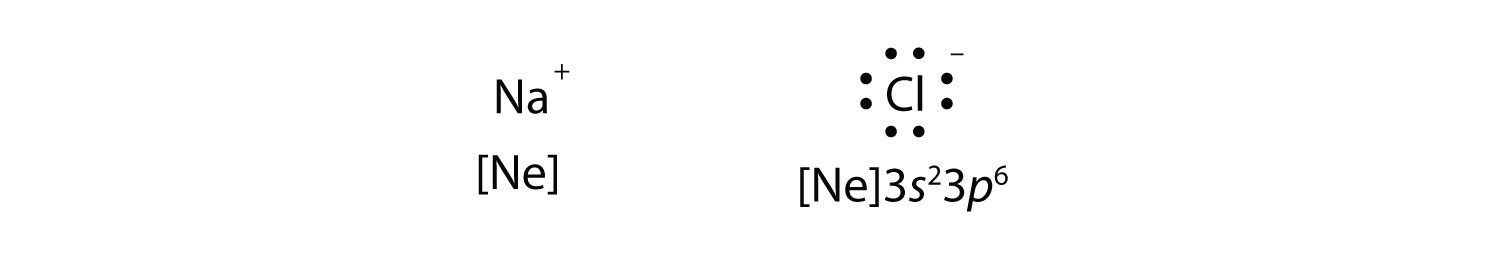






![Draw an electron dot diagram to show the formation of each of the following compounds:Magnesium Chloride. [H = 1, C = 6, Mg = 12, Cl = 17] .](https://i.ytimg.com/vi/_kgWhEiMDxQ/maxresdefault.jpg)



0 Response to "41 nacl lewis dot diagram"
Post a Comment