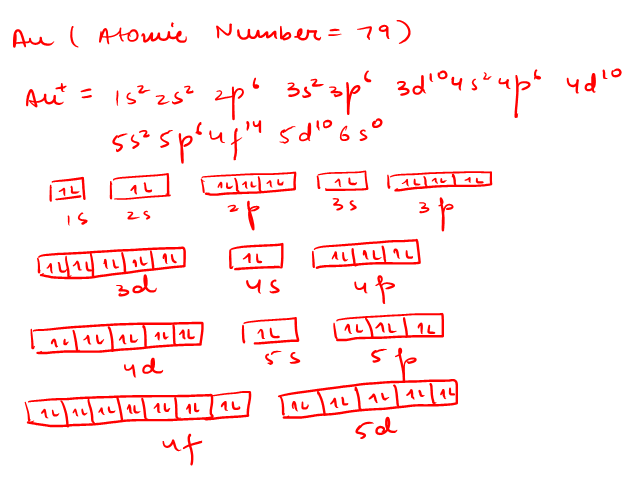
42 au+ orbital diagram
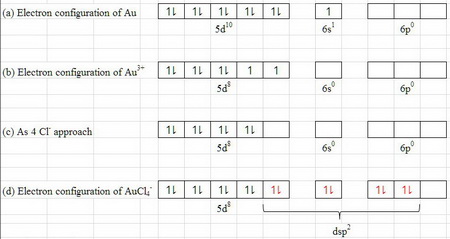
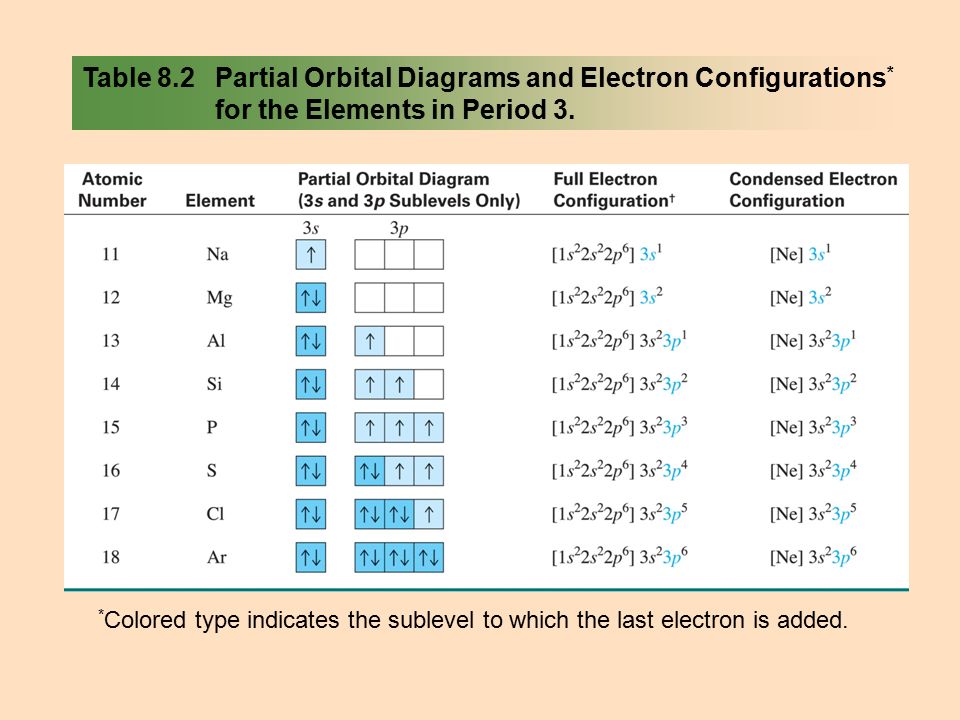
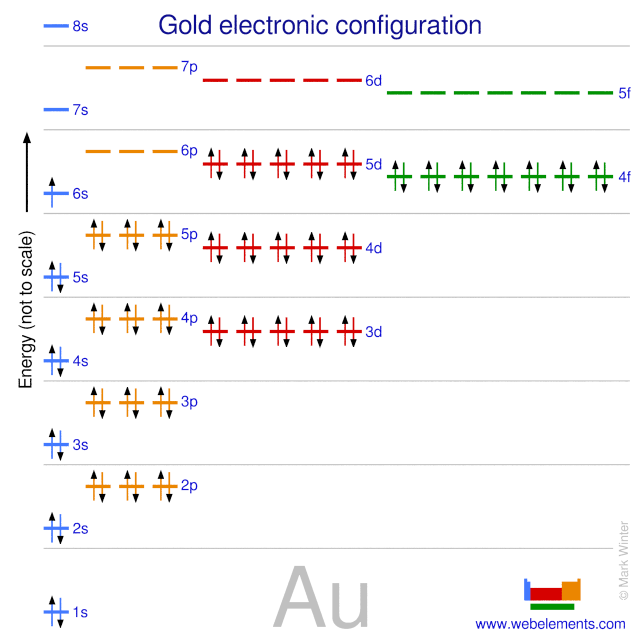
SOLVED:Write orbital diagrams for each ion and determine ... Problem 78 Hard Difficulty. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Orbital Diagram Au+ the atomic number of au is therefore, its for au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.orbital diagrams of atoms diagram shows how the electrons are distributed …
(Get Answer) - Write orbital diagram for Au+. Determine if ... Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Posted one year ago. Q: Write the abbreviated electron configuration and construct the orbital diagram for the chromium(II) ion. Is the ion paramagnetic or diamagnetic? ...
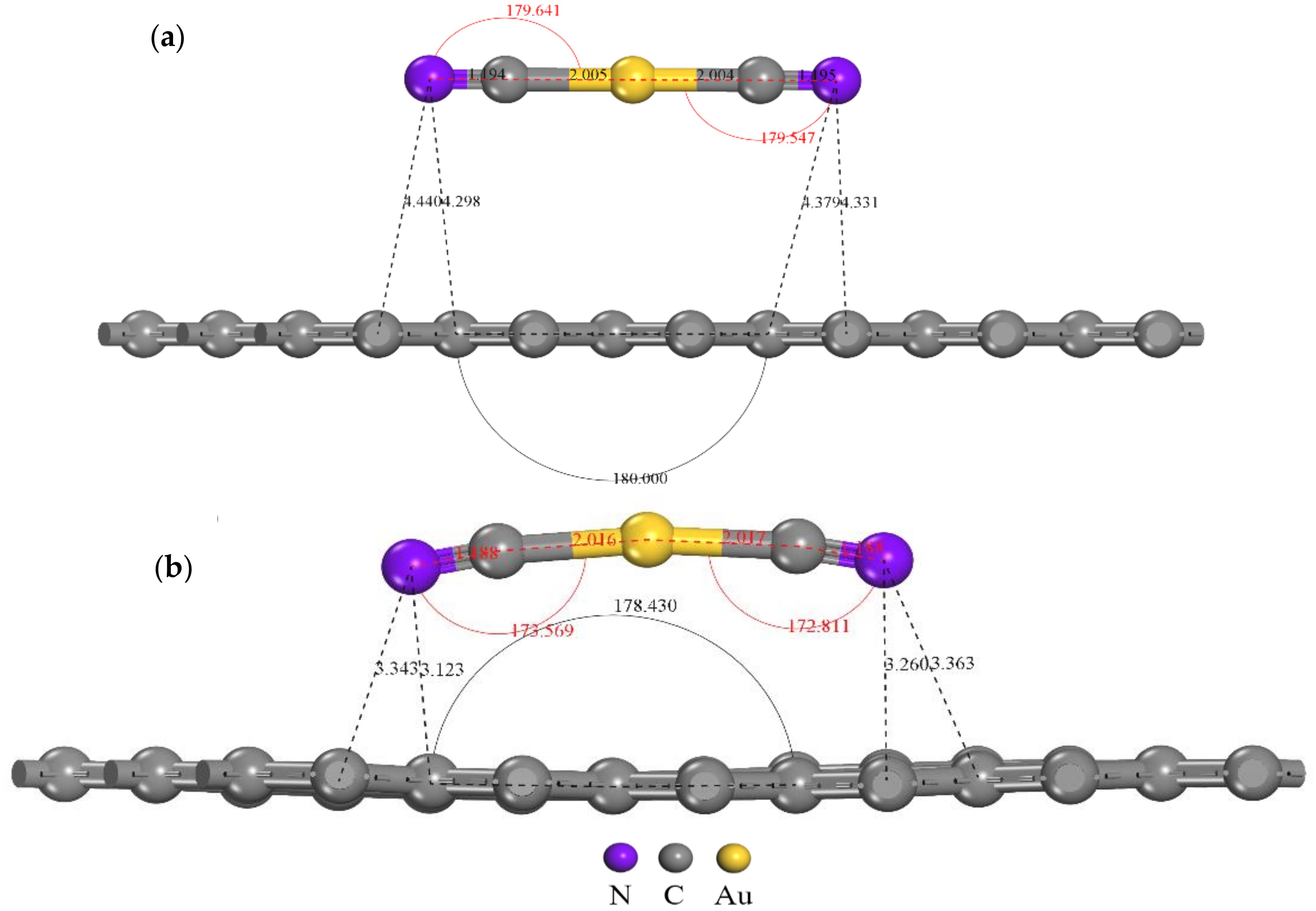
Au+ orbital diagram
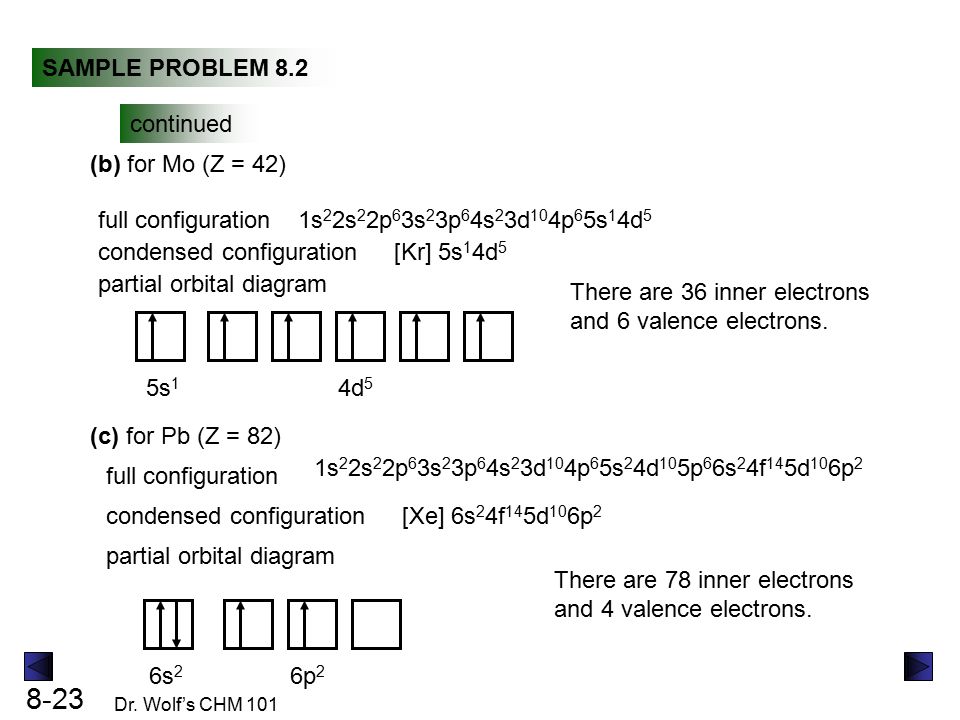
Write orbital diagram for Au+? - TheBasicAnswers.com Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital. What is the electron configuration of Au+? | Socratic Jan 3, 2016 — [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 ...1 answer · [Xe]4f145d10 Explanation: The atomic number of Au is 79. Therefore, its configuration is: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1 or, [Xe]4f145d106s ... Give the orbital diagram for Au+. | Study.com Answer to: Give the orbital diagram for Au+. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can also...
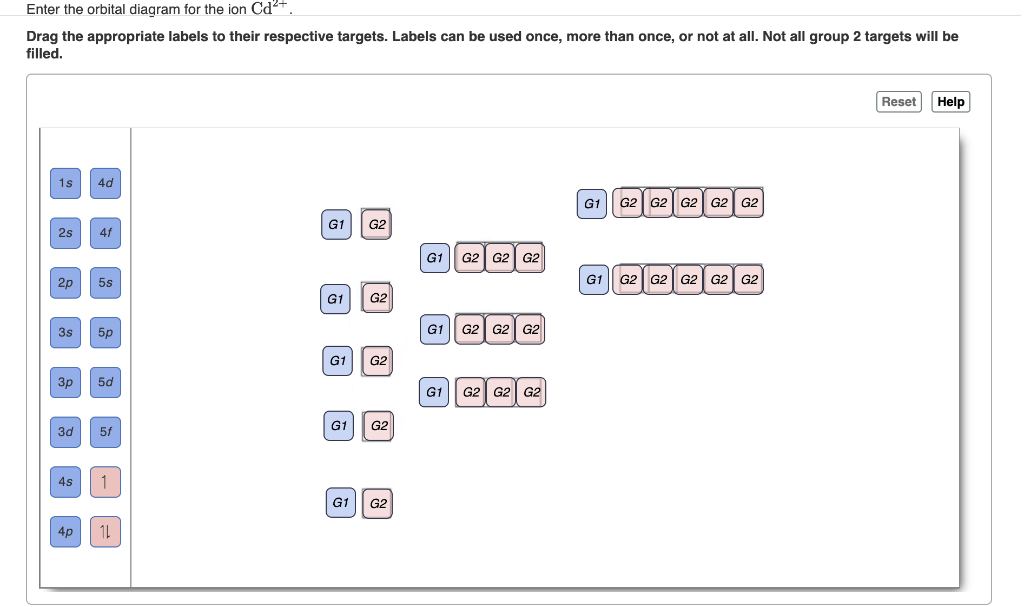
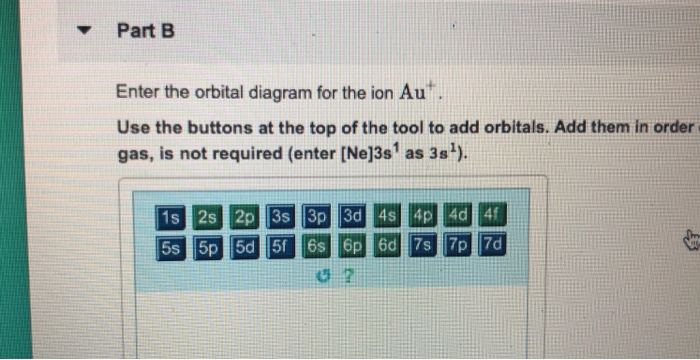
Au+ orbital diagram. Orbital Diagram For Au+ - Wiring Diagrams A) Write orbital diagram for Au+. B) Write orbital diagram for Zr2+. Expert Answer. What is the electron configuration of Au+? Get this answer with Chegg Study View this answer. Previous question Next question. Need an extra hand? Browse hundreds of Chemistry tutors. Nov 23, · which orbitals in the molecular orbital diagram contain the lone ... Excercise 14.13 question d - CHEMISTRY COMMUNITY Remember you base your cell diagram on your oxidation and reduction half reactions, so the LHS would be your anode (oxidation rxn) and your RHS would be your cathode (reduction reaction). You might've thought of it that way because you wanted to use Au+ -> Au3+ + 2e- for your oxidation reaction. Hope I helped. Answered: Write orbital diagrams for each ion and… | bartleby Solution for Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic.a. Cd2 + b. Au+ c. Mo3 + d. Zr2 + 40 enter the orbital diagram for the ion cd2+. - Diagram ... Enter the orbital diagram for the ion Au+ Enter the orbital diagram for the ion au+. An orbital diagram is the pictorial representation of shells in an atom by using square boxes (one box for s-orbital, three boxes for p-orbitals, five boxes for d-orbitals and seven boxes for f-orbitals) those boxes are filled by electrons using the following ...
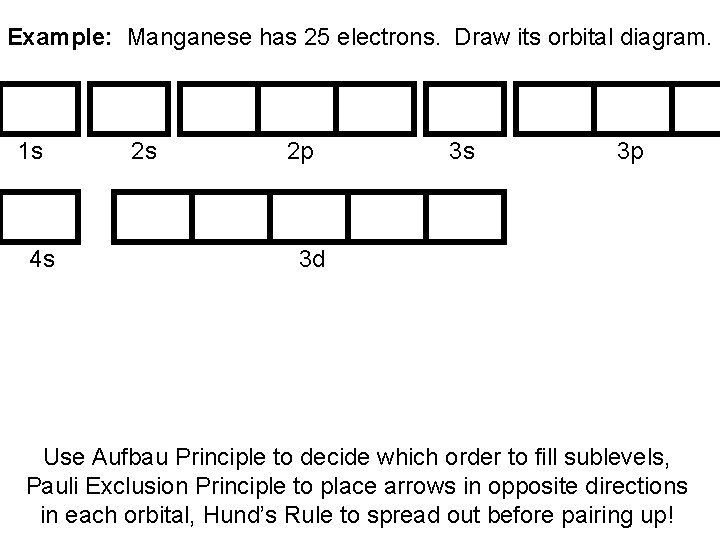
Solved Write orbital diagram for Au+. Determine if the ion ... See the answer See the answer done loading. Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Previous question Next question. what subshell contains only one orbital? | page 3 what is the orbital diagram for Au+, how do you fit the f orbitals in? Physics . 1) A planet travels in its orbit close to apogee, and in 2 years, its radius vector sweeps out an area of 3 A. How long will it take the planets radius vector to sweep an area of 3 A when it's close to perigee? A) a smaller time because the planets . Chemistry What is the orbital diagram for Au? - Answers Definition of orbital diagram? An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and... Enter the orbital diagram for the ion au+. An orbital diagram is the pictorial representation of shells in an atom by using square boxes (one box for s-orbital, three boxes for p-orbitals, five boxes for d-orbitals and seven boxes for f-orbitals) those boxes are filled by electrons using the following principles: Aufbau principle, Hund's rule, and Pauli's exclusion principle. Fundamentals
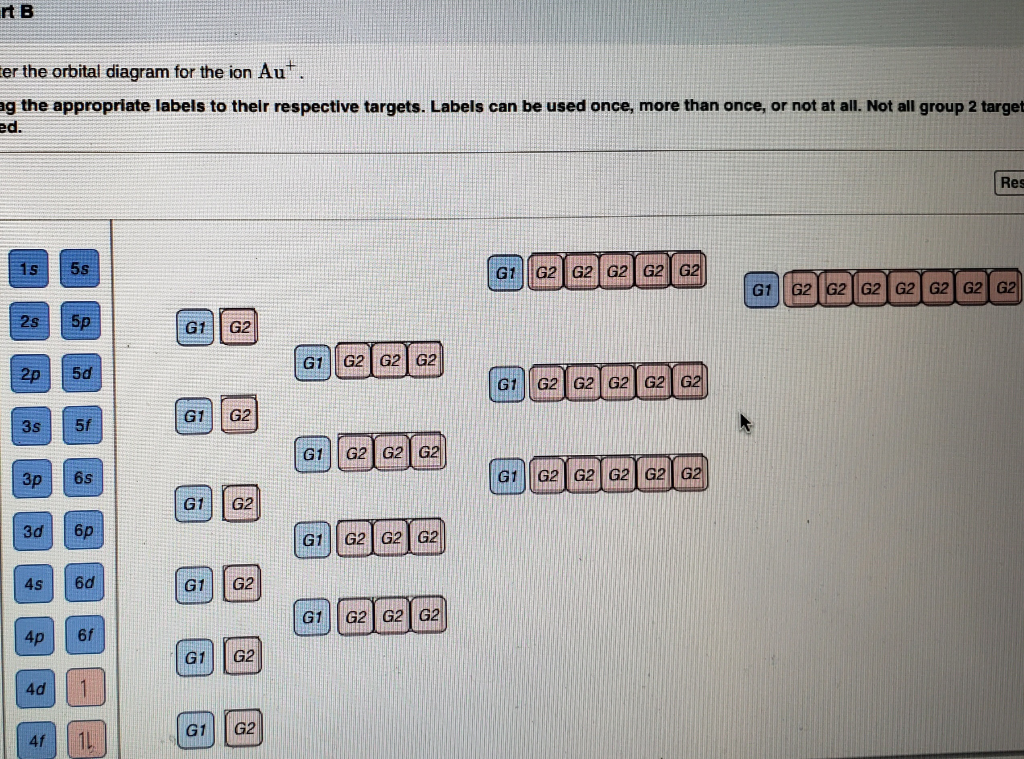
Solved Enter the orbital diagram for the ion Cd2+ Drag the ... Enter the orbital diagram for the ion Au+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Construct the orbital diagram for the ion Mo3+. Drag the appropriate labels to their respective targets. (Get Answer) - Write orbital diagram for Zr2+. Write ... 1. Enter the orbital diagram for the ion Cd2+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Enter the orbital diagram for the ion Au+. Use the... 44 enter the orbital diagram for the ion mo3+. - Wiring ... 2. Enter the orbital diagram for the ion Au+ 3.Construct the orbital diagram for the ion Mo3+ 4.Construct the orbital diagram for the ion Zr2+ ) Question: 1. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be ... Write orbital diagram for Au+? Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital.
Isometry Definition - What it is, Meaning and Concept ... Read More Write orbital diagram for Au+? Misc. Definition of viticulture - What it is, Meaning and Concept. By soetrust January 14, 2022. Gamers!!! Amazon Luna launches with freebies for Prime subscribersAmazon Luna special offer for Prime members!Try Amazon Luna Now!! It is known as viticulture to the set of techniques Y procedures that are ...
Chapter 8 Chemistry Homework Flashcards - Quizlet ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 4p6 4d10. Enter the orbital diagram for the ion Au+. ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level. 1.) Remove one electron from 5s1. ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
Orbital Diagram For Au+ - schematron.org Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons.
39 construct the orbital diagram of the f ion - Diagram ... Orbital Diagram For Au+ - schematron.org Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons.
SOLVED:Write orbital diagrams for each ion and determine ... Chapter three problems, seventy eight says to right orbital diagrams for several ions and determined at those ions. Our dia magnetic appear magnetic. So let's first go over those terms. If something is para magnetic, that means it has unpaid elections is that also means that it's slightly attracted to a magnetic field. If something is dia magnetic, that means all the electrons repairs.
Draw the orbital diagram for Au+. | Study.com Graphical representation of a molecule is done by following three rules, the Aufbau Principle, Hund's rule and the Pauli-Exclusion principle is termed as a ...1 answer · Top answer: The give cation is Au+Au+ named as gold ion. The atomic number in periodic table is 79. Electronic configuration...
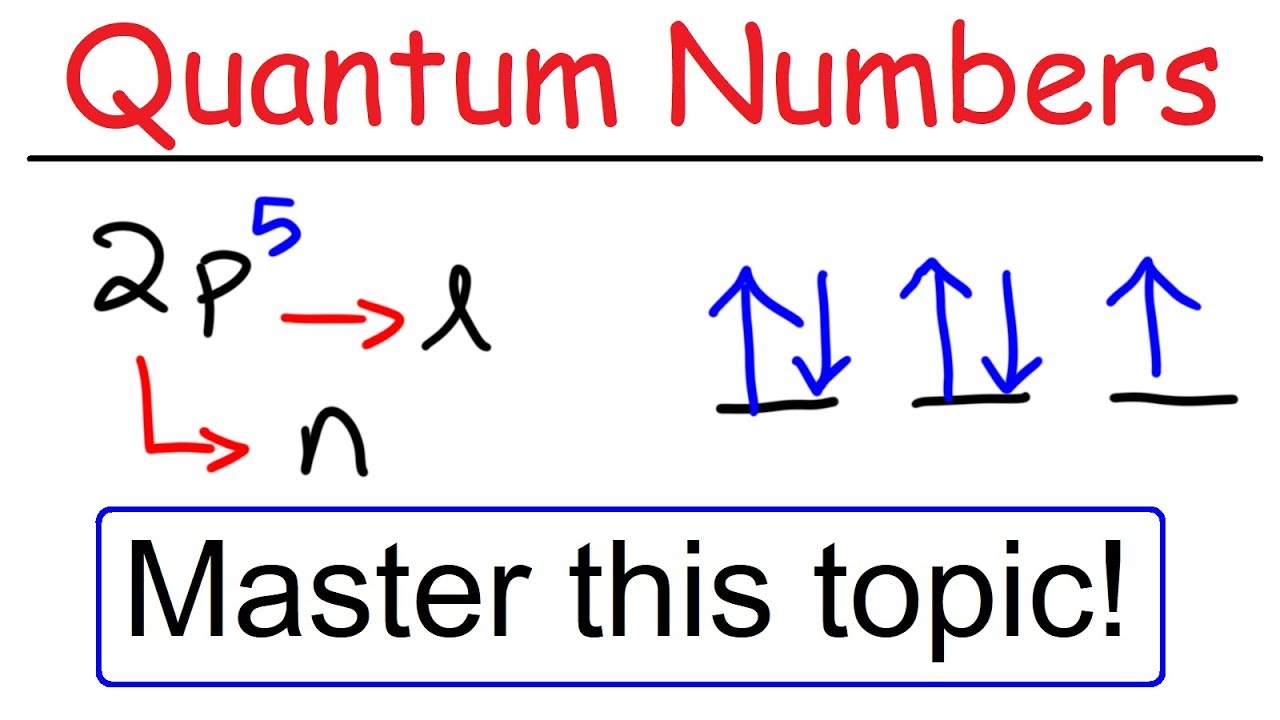
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Molecular orbital diagram of h2 - Soetrust Construct the molecular orbital diagram for he2; Use the molecular orbital diagram shown to determine which… using the molecular orbital theory, describe the bonding in… Write orbital diagram for Au+? Decide if N2 and N2+ are paramagnetic or diamagnetic. Which… What is the bond order of C2 2- ?
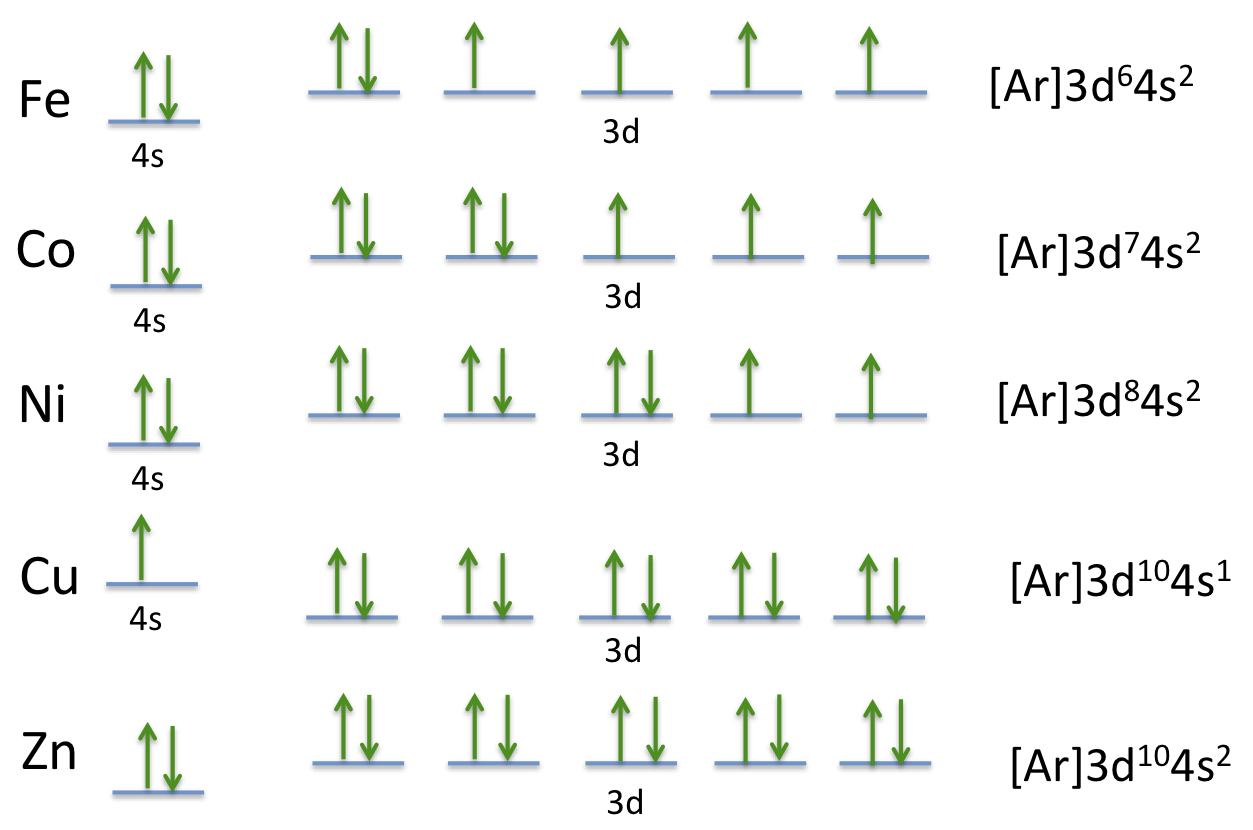
Enter The Orbital Diagram For The Ion Mo3+. Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., .
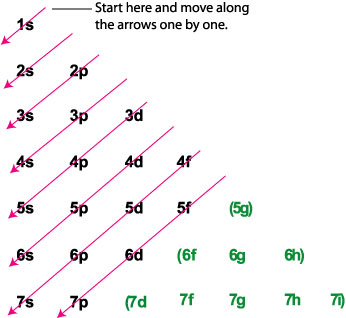
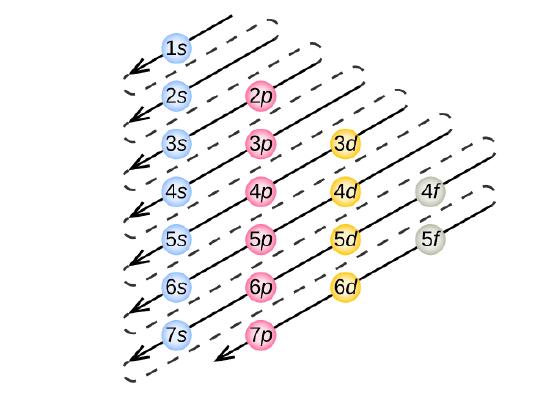
Enter The Orbital Diagram For The Ion Au+., Write Orbital ... So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the ...
what is the orbital diagram for Au+, how do you fit the f orbitals ... 1) The letter "p" in the symbol 4p^3 indicates the ___. A) spin of an electron B) orbital shape*** C) principal energy level D) speed of an electron 2) If the spin of one electron in an orbital is clockwise, what is the spin of . chemistry. Build the orbital diagram for the ion most likely formed by phosphorus.
Give the orbital diagram for Au+. | Study.com Answer to: Give the orbital diagram for Au+. By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can also...
What is the electron configuration of Au+? | Socratic Jan 3, 2016 — [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 ...1 answer · [Xe]4f145d10 Explanation: The atomic number of Au is 79. Therefore, its configuration is: 1s22s22p63s23p63d104s24p64d105s25p64f145d106s1 or, [Xe]4f145d106s ...
Write orbital diagram for Au+? - TheBasicAnswers.com Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital.



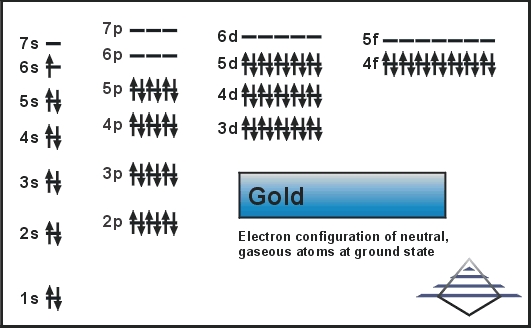









0 Response to "42 au+ orbital diagram"
Post a Comment